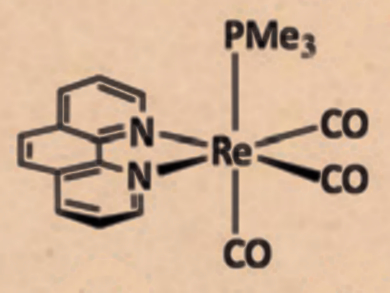
Among the most important donor ligands in transition-metal chemistry are pyridine-type ligands, 2,2′-bipyridine (bipy) and 1,10-phenanthroline (phen) in particular. The reactivity of these ligands upon coordination to transition metals has not been well-studied. Specifically, nucleophilic additions to the pyridyl ring of transition-metal-coordinated bipy or phen have not been reported.
Julio Pérez and colleagues, Universidad de Oviedo, Spain, have investigated various nucleophilic additions to rhenium-coordinated phen and bipy. Using the complex Re(CO)3(x)(PMe3)]OTf (x = phen or bipy), inter- and intramolecular additions were found. Upon reacting the phen complex with KN(SiMe3)2, which is usually non-nucleophilic in nature, this amide reversibly adds to the phen ligand leading to the intramolecular addition of the PMe3 ligand to phen.
Intermolecular additions were also found by reacting the Re complex, bearing either a phen or a bipy ligand, with MeLi. Interestingly, the amide was found to reversibly undergo addition, whereas MeLi reacted irreversibly. These reactions contribute to the range of possible reactivity pathways for such phen- and bipy-containing transition-metal complexes, providing insight into their deactivation in related studies.
- Nucleophilic Additions to Coordinated 1,10-Phenanthroline: Intramolecular, Intermolecular, Reversible, and Irreversible,
Rebeca Arévalo, M. Isabel Menéndez, Ramón López, Isabel Merino, Lucía Riera, Julio Pérez,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604438