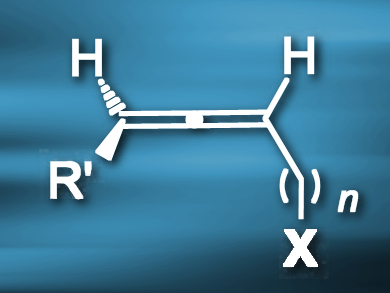
One of the earliest reported gold(I)-catalyzed reactions, the intermolecular hydroalkoxylation of allenes, suffers from poor chirality transfer. This is caused by the rapid racemization of the 1,3-disubstituted allene substrate (example pictured) under the reaction conditions.
Ai-Lan Lee and colleagues, Heriot-Watt University Edinburgh, UK, have investigated reaction conditions (i.e., solvent, temperature, nucleophile, and substrate scope) to improve the chirality transfer for these challenging substrates. They discovered that the use of a coordinating solvent such as dimethylformamide in combination with low temperatures of approximately 0 °C helped to significantly decrease allene racemization. Furthermore, the addition of higher equivalents of alcohol nucleophile (e.g., BnOH) improved the enantiomeric ratio of the hydroalkoxylated products.
A wide variety of allene substrates was tolerated in this reaction, including sterically hindered and acid-sensitive allenes. Some functional groups on the substrate were actually necessary to achieve excellent regioselectivities through inductive withdrawing effects. With this protocol, an excellent chirality transfer of up to 99:1 enantiomeric ratio could be achieved.
- Chirality Transfer in Gold(I)-Catalysed Hydroalkoxylation of 1,3-Disubstituted Allenes,
Stacey Webster, Daniel R. Sutherland, Ai-Lan Lee,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201603918