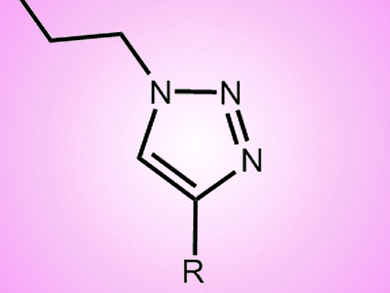
Functionalized triazoles have recently been identified as the lead targets for potential pharmaceutical drugs. Carolynne Ricardo and Tomislav Pintauer, Duquesne University, Pittsburgh, USA, have developed a one-pot reaction using click chemistry to produce a range of functionalized triazoles. They employ sequential azide–alkyne [3+2] cycloaddition and an atom transfer radical addition, both of which are catalyzed by copper.
Tris(2-pyridylmethyl)amine (TPMA) was used in the presence of ascorbic acid as an environmentally benign reducing agent and the catalyst loading could be as low as 0.5 mol%. Reactions with azidopropyl methacrylate and 1-(azidomethyl)-4-vinylbenzene in the presence of a variety of alkynes and alkyl halides proceeded efficiently to yield highly functionalized (poly)halogenated esters and aryl compounds containing a triazolyl group
The introduction of halogen functionality opens up the possibilities for further transformations of triazoles such as elimination, displacement, conversion to a Grignard reagent, etc. Also, the halogen functionality could serve as further radical precursor.
.gif)