Weinreb amides are synthetically useful organic compounds and have recently gained popularity as directing groups. Efforts have been directed towards C–H functionalization of the amides without loss of the N-alkoxy moiety. Rhodium and palladium-catalyzed oxidative Heck processes with olefins in which the N-alkoxy group tolerates the reactions conditions have been developed.
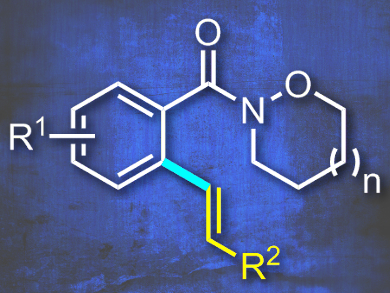
Riki Das and Manmohan Kapur, Indian Institute of Science Education and Research, Bhopal, India, have found a new class of cyclic Weinreb amides that can be used in Ru-catalyzed oxidative Heck reactions. Previous work from the authors using an identical catalyst system provided products with cleaved N-alkoxy moieties. The amides in this current work kept their synthetic feature intact simply by applying larger 6-and 7-membered cycles (example pictured). The undesired N–O reductive bond cleavage was thus inhibited and the target C–H olefination products were obtained.
The reaction was found to be site-selective, tolerant of a diverse amount of substrates, and provided only the monoolefination products in good yields without any diolefination. Kinetic and deuterium-labelling studies provided some mechanistic insight; the formation of a ruthenacycle is reversible and the reaction is pushed forward by the faster rate of all subsequent steps. The team demonstrates the synthetic utility of the products with further functionalization.
- Product Control using Substrate Design: Ruthenium-Catalysed Oxidative C−H Olefinations of Cyclic Weinreb Amides,
Riki Das, Manmohan Kapur,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201603126