The synthesis of pyrroles and their derivatives has attracted much attention because of their prevalence in natural products, agrochemicals, flavors, dyes, materials, and drugs. Traditional methods to prepare pyrroles suffer from drawbacks such as waste generation, multistep syntheses, and poor availability of starting materials. Progress has been made in sustainable pyrrole synthesis using noble-metal-based catalysts. However, the development of nonprecious metal catalysts is desirable, from the perspectives of abundance, cost, and toxicity.
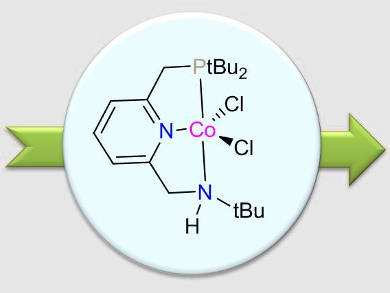
David Milstein and colleagues, Weizmann Institute of Science, Rehovot, Israel, have developed a pyrrole synthesis catalyzed by a base-metal complex. It involves the acceptorless dehydrogenative coupling of 1,4-substituted butanediols and various primary alkyl, benzyl, and aryl amines using a cobalt pincer complex (pictured) to generate 1,2,5-substituted pyrroles. Water and hydrogen gas are the only by-products of the reaction.
A unique feature of the used pincer complexes is that they have the potential for metal–ligand cooperation by both amine–amide and aromatization–dearomatization ligand transformations. According to the researchers, further investigation regarding the mechanism of this reaction is underway.
- Direct Synthesis of Pyrroles by Dehydrogenative Coupling of Diols and Amines Catalyzed by Cobalt Pincer Complexes,
Prosenjit Daw, Subrata Chakraborty, Jai Anand Garg, Yehoshoa Ben-David, David Milstein,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201607742