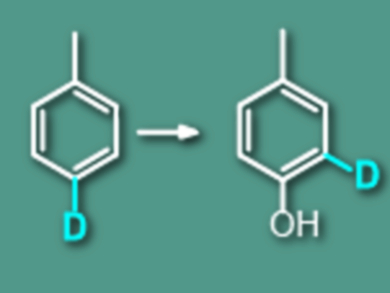
The hydroxylation of weakly polar aromatics into water-soluble phenols can be performed by advanced oxidation technologies such as aqueous TiO2 photocatalysis and the Fenton reaction. In many cases, this is the only route for the degradation of these insoluble aromatic pollutants in water. However, the mechanism by which the hydroxyl group finally attaches to the benzene ring has remained tricky.
Jincai Zhao and Wanhong Ma, Chinese Academy of Sciences, Beijing, used deuterium-labeled toluene and p-xylene as model substrates to observe the replacement of ipso-2H and CH3 by OH groups by aqueous TiO2 photocatalysis and the Fenton reaction.
The team found found that in the hydroxylation products, a certain proportion of 2H and CH3 groups was not replaced by OH groups as expected in the previously proposed O2-insertion mechanism. Instead, they were shifted to the adjacent carbon position of the ring (called a 1,2-NIH shift effect).
The results indicate that there is more than one mechanism to attach the OH group of H2O or H2O2 onto the benzene ring.
- Mechanistic Studies of TiO2 Photocatalytic and Fenton Degradation of Hydrophobic Aromatic Pollutants in Water,
Jincai Zhao, Wanhong Ma,
Chem. Asian J. 2016.
DOI: 10.1002/asia.201601299