Fuel cells are electrochemical energy converters. They directly convert the energy of a chemical reaction into electrical energy – without a thermal-electric intermediate step.
General Principle
In an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between the reacting molecules or atoms. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat.
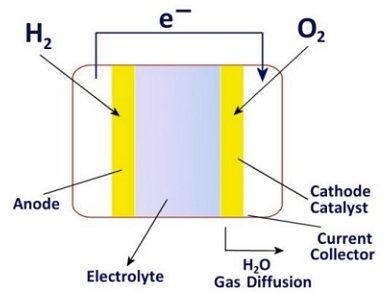
In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. The electrons then flow to the cathode where they are taken up by the other reaction partner, typically atmospheric oxygen.
By this, the reaction can be controlled and carried out with high current efficiency.
Design
Fuel cells consist of two electrodes that conduct electrons – the anode and the cathode. The electrodes are separated by an electrolyte that conducts ions.
In a battery the electrical energy is chemically stored in the electrode. In a fuel cell the energy source is brought in from externally. The electrodes only have a catalytic role in the conversion.
The most simple and favored reactants are hydrogen as fuel and oxygen as oxidant. Also hydrocarbons such as natural gas and alcohols like methanol are used as fuels.
In technical systems fuel cells are coupled to stacks to achieve higher electrical voltage and meet demand. Fuel cell systems have a high efficiency which, in principle, can outperform those of combustion engines.
Types
High/Low Temperature Fuel Cells
Fuel cells are subdivided in low temperature (LT) and high temperature (HT) fuel cells. LT fuel cells work in a range from room temperature to approx. 120 °C and HT fuel cells from approx. 600 to 1,000 °C. The phosphoric acid fuel cell operates at a temperature in between, at 200 °C.
Electrolytes Used
Fuel cells are usually named according to the electrolyte used. The different electrolytes significantly affect the characteristic properties of the fuel cells like operating temperature and mechanism of conductivity. Directly related to this is the selection of the catalyst, demand of the process gas, etc.

1LT fuel cells require precious metal catalysts in their electrodes to activate the chemical reaction. This makes them sensitive to CO poisoning at low temperatures. The higher operating temperature of PAFC tolerates CO concentrations of 1–2 %. Instead of pure hydrogen, also process gas can directly be used.
2PEMFC use a polymer as electrolyte.
3The name DMFC refers to the direct conversion of methanol as fuel. Typically it works on the basis of a PEMFC.
References
Modified translation from
M. Waidhas, H. Landes, Gebändigtes Knallgas: Brennstoffzellen im mobilen und stationären Einsatz, Phys. Unserer Zeit 2001, 32(4), 172–179. DOI: 10.1002/1521-3943(200107)32:4<172::AID-PIUZ172>3.0.CO;2-W