Iodine Test
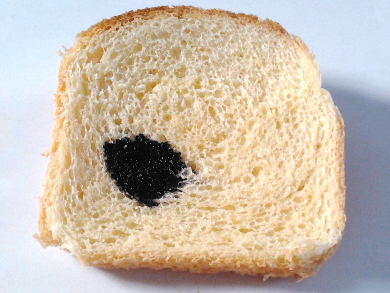
Using iodine to test for the presence of starch is a common experiment. A solution of iodine (I2) and potassium iodide (KI) in water has a light orange-brown color. If it is added to a sample that contains starch, such as the bread pictured above, the color changes to a deep blue. But how does this color change work?
Starch is a carbohydrate found in plants. It consists of two different types of polysaccharides that are made up of glucose units which are connected in two different ways. One is the linear amylose and the other is the branched amylopectin (pictured below).
 |
Amylose is the compound that is responsible for the blue color. Its chain forms a helix shape, and iodine can be bound inside this helix (pictured below).
.jpg) |
Charge-Transfer Complexes
The colors are caused by so-called charge transfer (CT) complexes. Molecular iodine (I2) is not easily soluble in water, which is why potassium iodide is added. Together, they form polyiodide ions of the type In–, for example, I3–, I5–, or I7–. The negatively charged iodide in these compounds acts as charge donor, the neutral iodine as a charge acceptor. Electrons in such charge-transfer complexes are easy to excite to a higher energy level by light. The light is absorbed in the process and its complementary color is observed by the human eye.
In the case of the aqueous solution of polyiodides, the absorptions of the different species lead to an overall brownish color. Once amylose is added, it forms another CT complex, Here, the amylose acts as a charge donor and the polyiodide as an acceptor. This complex absorbs light of a different wavelength than polyiodide, and the color turns dark blue.
Polyiodide Chains
The exact structure of the polyiodides inside the amyloid helix is not clear. The amylose-iodine complex is amorphous (i.e., it does not form ordered crystals), which has made it difficult to determine its structure. It has been proposed that the species inside the helix are repeated I3– or I5– units.
However, Ram Seshadri, Fred Wudl, and colleagues, University of California, Santa Barbara, USA, have found evidence that infinite polyiodide chains Inx– are contained in the amylose-iodine complex [1]. The team investigated a related system, a pyrroloperylene–iodine complex, to study its properties as an organic electronic conductor. The material is crystalline, and therefore, the team was able to determine its structure using X-ray crystallography. They found nearly linear polyiodide chains in-between stacks of pyrroloperylene. It turned out that the material containing these chains absorbs light at very similar wavelengths to the amylose-iodine complex, which supports the hypothesis that similar polymeric chains form in the iodine test for starch.
Reference
[1] Sheri Madhu, Hayden A. Evans, Vicky V. T. Doan-Nguyen, John G. Labram, Guang Wu, Michael L. Chabinyc, Ram Seshadri, Fred Wudl, Infinite Polyiodide Chains in the Pyrroloperylene-Iodine Complex: Insights into the Starch-Iodine and Perylene-Iodine Complexes, Angew. Chem. Int. Ed. 2016, 55, 8032–8035.
DOI: 10.1002/anie.201601585
Sources
- Der Iod-Stärke-Komplex (in German),
www.chemieunterricht.de 2006.
(accessed November 24, 2016) - The structure of the blue starch-iodine complex,
Wolfram Saenger,
Naturwissenschaften 1984, 71, 31–36.
DOI: 10.1007/bf00365977


this article is quite helpful .. thank you ✨
Thank you guys
where the h*** is the volume and page number to cite this
Dear Reader,
We do not have volumes or page numbers. You can cite the article as: C. Goedecke, Why Does Iodine Turn Starch Blue?, ChemistryViews 2016. https://doi.org/10.1002/chemv.201600103
Best regards, Your ChemistryViews Team
Interesting light absorbing properties
Nice informative post
Very informative thank you very much
Who is the publisher?
Dear Reader,
You can cite the article as: C. Goedecke, Why Does Iodine Turn Starch Blue?, ChemistryViews 2016. https://doi.org/10.1002/chemv.201600103
Best regards, Your ChemistryViews Team
Very interesting and helpful
Very helpful imformation, thank you
Wow that’s awesome
this is awesome
really cool but no words
would iodiDE alone form the same reaction? or is iodiNE specifically needed to react with the glycogen
Thanks for this.. very helpful
What does it mean, when the water become crystal clear after you added iodine into the water?
Nice 👍👍
grazie, molto chiaro