On-surface chemistry is an interesting research area. Often, unprecedented structures can be synthesized on surfaces—for example, functional nanomaterials. Desilylative reactions involving C–Si bond cleavage are well-known in solution chemistry. In contrast to this, desilylation in on-surface chemistry is limited. There are known examples of C(sp)–Si cleavage, while C(sp2)–Si cleavage on a metal surface had not been achieved so far.
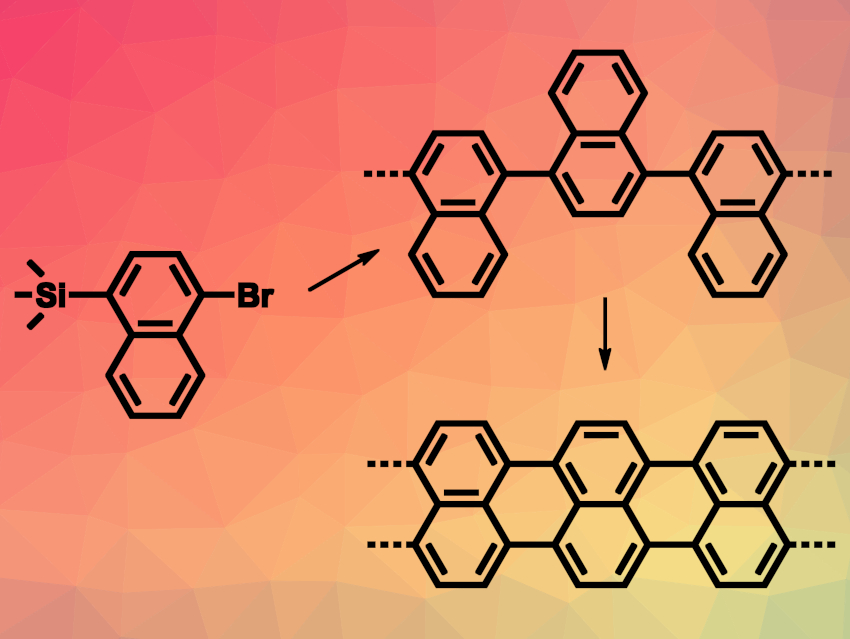
Zhixiang Sun, You Han, Hong-Ying Gao, Tianjin University, China, Dongbing Zhao, Nankai University, Tianjin, China, and colleagues have developed reactions that involve C(sp2)–Si bond cleavages on metal surfaces (pictured). The team tested four precursors for such reactions, i.e., 1-trimethylsilyl-4-bromo-naphthalene (1,4-TBN, pictured above), 2-trimethylsilyl-6-bromo-naphthalene (2,6-TBN), 1,4-bis(trimethylsilyl)naphthalene (1,4-BTN), and 1,4-dibromonaphthalene (1,4-DBN). The reactions were performed on Au(111), Ag(111), or Cu(111) surfaces.
Using high-resolution scanning tunneling microscopy (STM), the team found that 1,4-TBN molecules form an ordered, self-assembled, chain-like structure when deposited on an Au(111) surface. Thermal annealing then led to the cleavage of C–Br and C(sp2)–Si bonds and C–C coupling reactions.
Annealing at 250 °C gave poly-naphthalene chains. Further annealing at 300 °C then led to 5-armchair graphene nanoribbons (5-AGNRs), formed by partial cyclodehydrogenation. According to the researchers, this is the first successfully realized C(sp2)–Si desilylation on a surface.
- Desilylative Coupling Involving C(sp2)–Si Bond Cleavage on Metal Surfaces,
Kang Ma, Tiantong Zhang, Ying Qin, Zhixin Hu, Zhixiang Sun, You Han, Dongbing Zhao, Hong-Ying Gao,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c02762