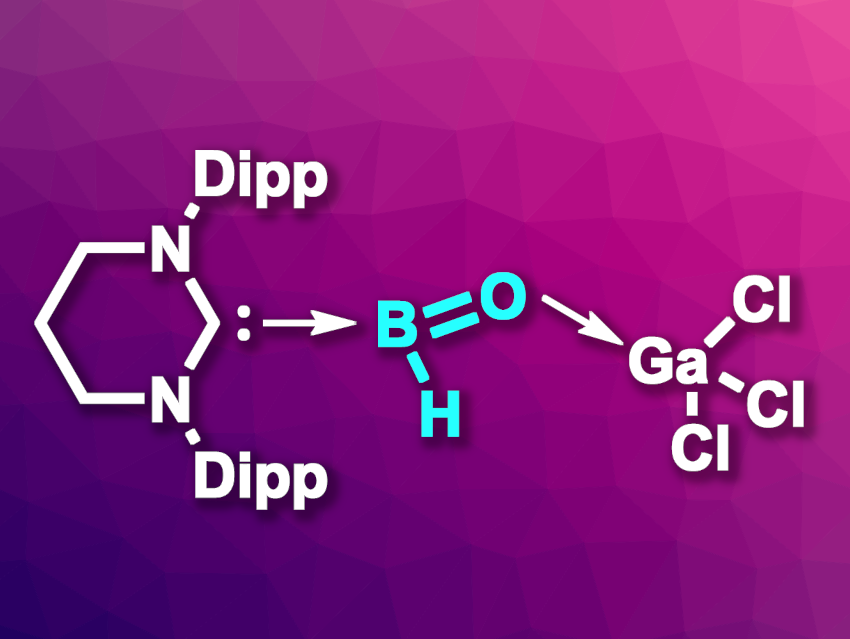
The parent oxoborane, H–B=O, has so far only been detected in the gas phase or in low-temperature matrices. Difficult-to-isolate species such as this can sometimes be stabilized using a donor/acceptor approach, i.e., using an electron-donating group on one side and an electron-accepting group on the other side.
Sakya S. Sen, CSIR-National Chemical Laboratory, Pune, India, and Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India, and colleagues have synthesized a rare acid-stabilized neutral derivative of the parent oxoborane (pictured). The compound has a strongly electron-donating N-heterocyclic carbene (NHC), i.e., 6-SIDipp (1,3-di(2,6-diisopropylphenyl) tetrahydropyrimidine-2-ylidene) on one side and GaCl3 as an electron-accepting Lewis acid on the other side. The team first synthesized an unusual boron gallium compound via a reaction of 6-SIDipp·BH3 with GaCl3.
The resulting boron gallium compound was reacted with water as an oxygen source to form the desired stabilized oxoborane. H2 was eliminated during this reaction. According to the researchers, the product can be considered as an acid-stabilized parent oxoborane, containing an unusual H(B=O) linkage. Adding more water to this oxoborane derivative leads to the release of another molecule of H2 and gives a hydroxy oxoborane derivative.
- Taming the parent oxoborane,
Sakya Singha Sen, Gargi Kundu, P. R. Amrutha, K. Vipin Raj, Srinu Tothadi, Kumar Vanka,
Chem. Sci. 2023.
https://doi.org/10.1039/D3SC01544K