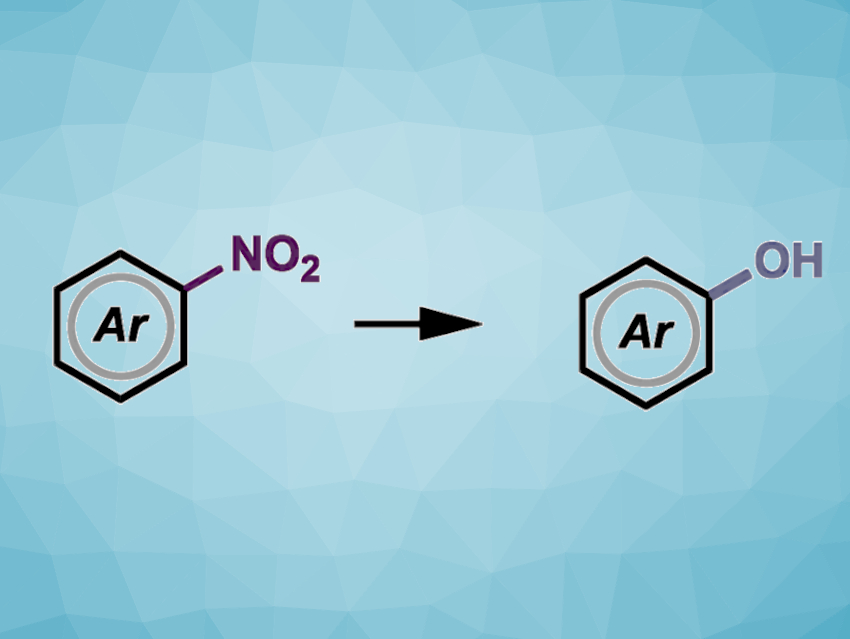
Nitroarenes are easily prepared, abundant chemical feedstocks and useful synthetic intermediates in organic chemistry. The nitro group can be converted into a large variety of functional groups through a well-established three-step sequence consisting of (1) reduction, (2) diazotization, and (3) substitution through Sandmeyer-type reactions. However, this approach typically requires multiple synthetic steps, harsh reaction conditions, and the handling of hazardous intermediates.
Michael James, The University of Manchester, UK, and colleagues have developed a method to directly convert nitroarenes into phenols in one step and under transition-metal-free conditions. The team reacted a variety of nitroarenes with an easily prepared oxime reagent (pictured below), potassium hydroxide as a base, and dimethyl sulfoxide (DMSO) as the solvent. The reactions were performed at 85 °C. The researchers found that electron-deficient substrates react at lower temperatures.
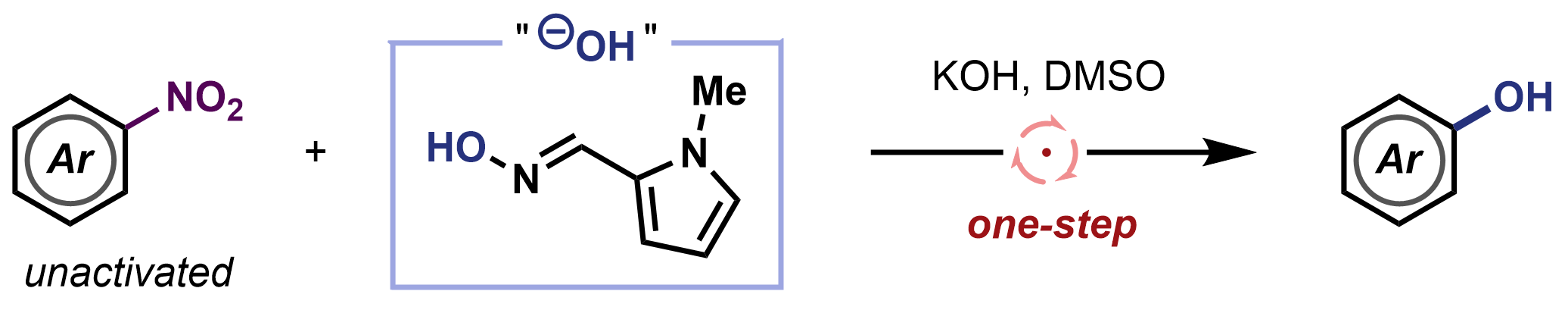
The team proposes an electron-catalyzed SRN1 mechanism involving the fragmentation of nitroarene radical anions to form aryl radicals. The work could be useful for the development of other direct denitrative functionalization reactions.
- Denitrative hydroxylation of unactivated nitroarenes,
Lee Duff, Harry Meakin, Adam Richardson, Andrew J. Greener, George W. A. Smith, Ivan Ocaña, Victor Chechik, Michael J. James,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202203807