Metal-catalyzed cross-coupling reactions are widely used in organic synthesis. They typically require suitable functional groups on both reaction partners. C–H functionalization reactions avoid the need for prefunctionalization but can be more difficult to achieve due to the lower reactivity. Cross-coupling reactions that involve C–H activation on both substrates are, thus, rare.
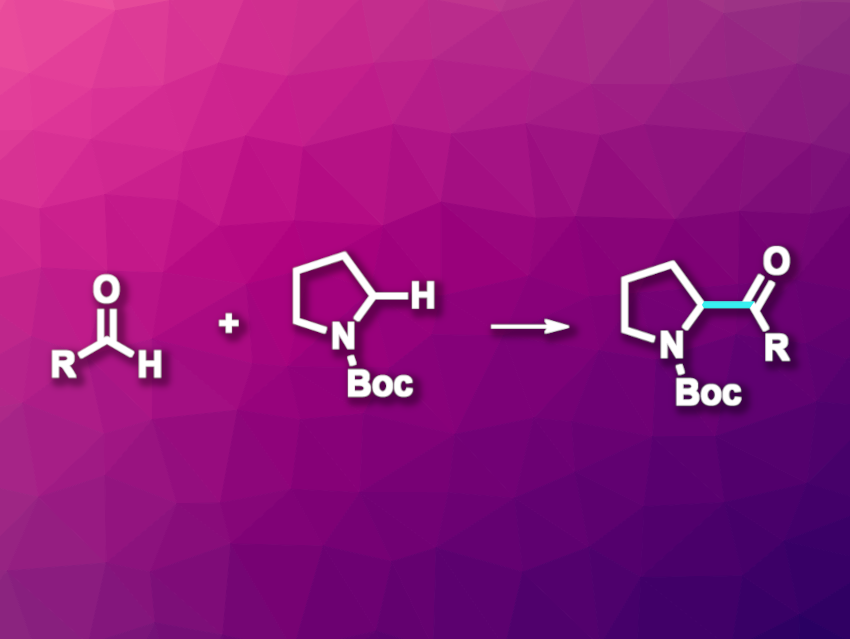
Paul M. Zimmerman, John Montgomery, University of Michigan, Ann Arbor, USA, and colleagues have developed an approach to an oxidative, cross-selective dehydrogenative coupling of N-heterocycles with aldehydes that gives ketone derivatives (pictured). The team used NiBr2·dme or NiCl2·dme (dme = dimethoxyethane) as the catalyst together with bis(pyrazole) pyridine (bpp) as a ligand, di-tert-butyl peroxide (DTBP) as an oxidant, zinc as a reductant, and MeCN as the solvent. The reactions were performed at 50 °C.
Under these conditions, different aldehydes were coupled with a variety of protected nitrogen heterocycles. Tetrahydrofuran and acyclic systems with carbamate, urea, benzamide, or benzyl functionalities were also found to be suitable substrates. The desired ketones were obtained in moderate to good yields. The team proposes a reaction mechanism that involves the formation of radical species from both reaction partners.
- Oxidative Cross Dehydrogenative Coupling of N-Heterocycles with Aldehydes through C(sp3)–H Functionalization,
Mo Chen, Austin M. Ventura, Soumik Das, Ammar F. Ibrahim, Paul M. Zimmerman, John Montgomery,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c06532



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)