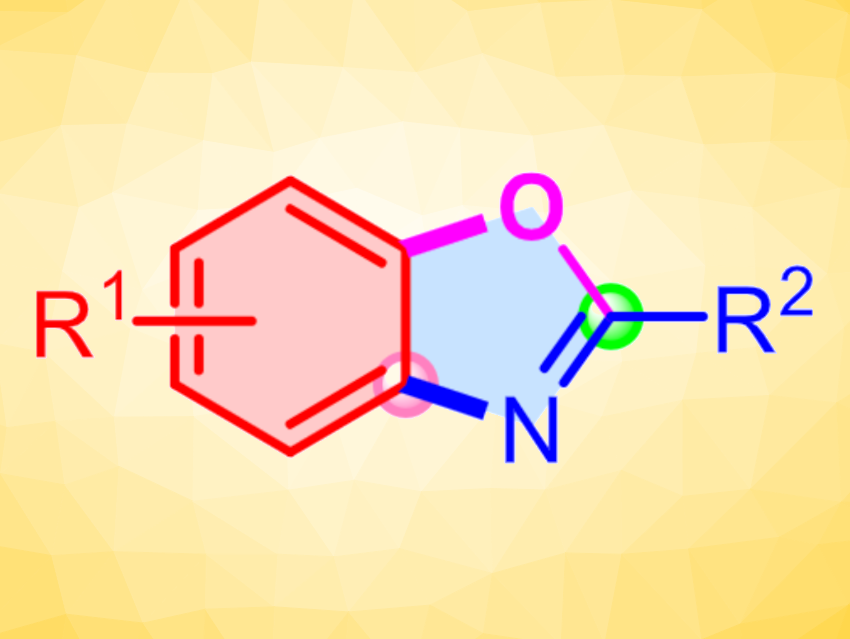
2-Substituted benzoxazoles are commonly found motifs in a wide range of bioactive compounds, ranging from natural products to pharmaceuticals. Usually, multistep procedures are required to prepare such heterocycles, and the scope of substrates available is often narrow. To meet the demands of the pharmaceutical industry, a general method to streamline the synthesis of 2-substituted benzoxazoles from a wide range of simple, readily available starting materials would be useful.
Weiping Su and Biping Xu, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, China, have developed a straightforward, transition-metal-free method for the synthesis of 2-substituted benzoxazoles from readily available cyclohexanones and aliphatic primary amines (pictured, 4 Å MS = 4 Å molecular sieve) via an imine α-oxygenation-initiated cascade reaction sequence. The team achieved high selectivity and functional-group tolerance when (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) was used as a mild oxidant that oxidized the reaction intermediates selectively.
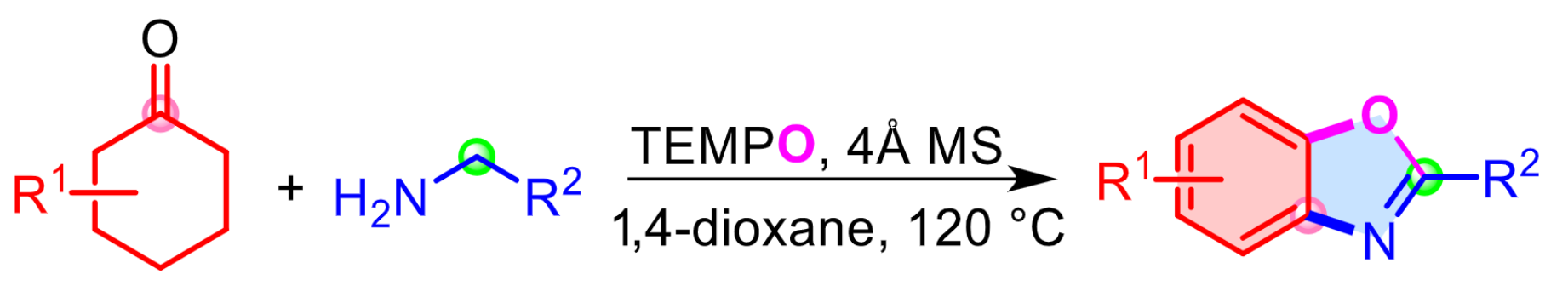
The method provides straightforward access to structurally complex drugs and natural products containing 2-substituted benzoxazole units that are unattainable by existing methods. The reaction can be performed on a gram scale and the reactants are readily available and low-cost.
- A Tandem Dehydrogenation‐Driven Cross‐Coupling between Cyclohexanones and Primary Amines for Construction of Benzoxazoles,
Biping Xu, Weiping Su,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202203365