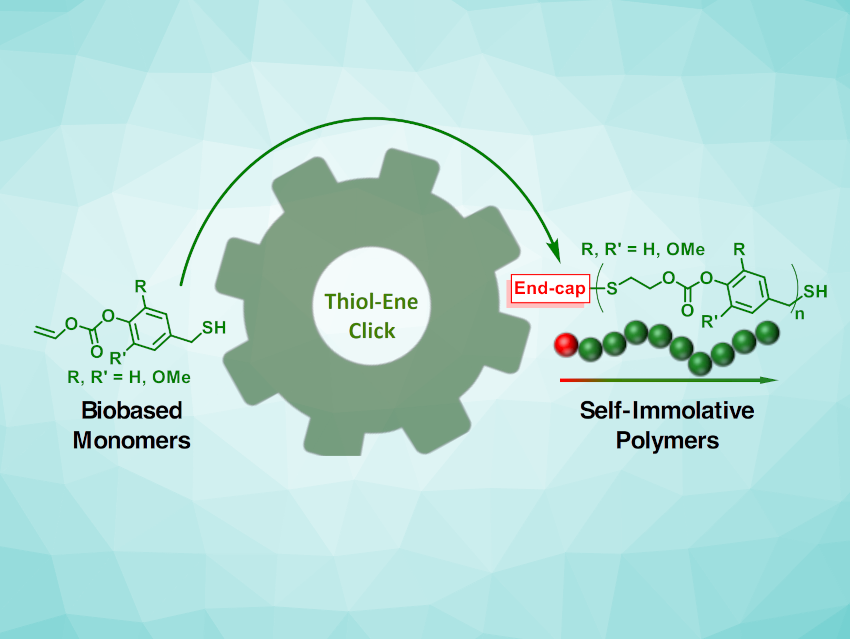
Polymer waste and the use of fossil resources for their synthesis are important environmental and sustainability issues. The development of polymers that can be made from renewable resources and that can undergo depolymerization after use are, thus, interesting research targets. Self-immolative polymers undergo end-to-end depolymerization in response to certain stimuli, which makes them useful in this context. However, combining this feature with the construction from renewable building blocks can be challenging.
Elizabeth R. Gillies, The University of Western Ontario, London, Canada, and colleagues have developed a new class of self-immolative polymers (pictured) that are based on renewable, lignin-derived aldehydes and can be synthesized via a simple thiol-ene polymerization. The team prepared bifunctional monomers with, e.g., allyloxycarbonyl and benzyl thiol subunits and combined them with acrylate-terminated poly(ethyleneglycol) (PEG-Acrylate) in block co-polymers.
The researchers introduced different thiol-containing stimulus-reactive end caps via thiol-ene reactions. The selective removal of the end caps allowed them to induce a cascade depolymerization, while the polymer remained stable in the absence of the respective stimuli. The work provides a path to biomass-derived polymers that can be degraded on demand.
- Self‐Immolative Polymers Derived from Renewable Resources via Thiol−Ene Chemistry,
Elizabeth Gillies, Zhengyu Deng,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202420054