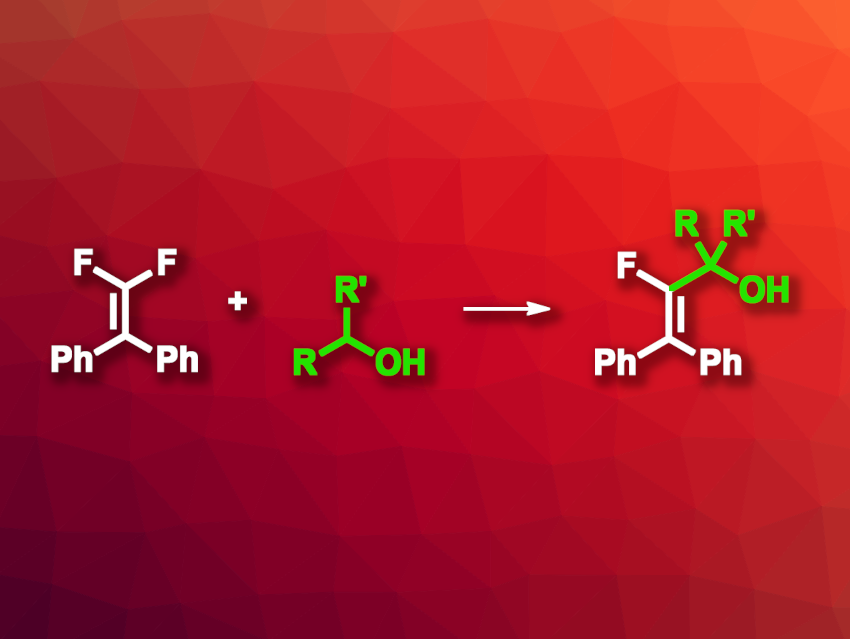
Monofluoroalkenes can be useful, e.g., in drug development, where they can serve as a replacement for peptide bonds. One synthetic path to monofluoroalkenes is the C–F activation of gem-difluoroalkenes. However, existing methods for this often have drawbacks such as a need for prefunctionalized starting materials or limited substrate scopes. Thus, new methods for the synthesis of alkylated monofluoroalkenes via C–H/C–F bond coupling could be useful.
Wengang Xu, Mingbo Wu, China University of Petroleum (East China), Qingdao, and colleagues have developed an effective method for the synthesis of monofluroalkenes—in particular, α-fluoroalkenyl alcohols—by the defluoroalkylation of gem-difluoroalkenes with alcohols via a C–F/C–H coupling (example reaction pictured). The team reacted different aryl-substituted gem-difluoroalkenes with a variety of alcohols under blue LED light, using 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) as a photocatalyst in the presence of quinuclidine, ZnCl2, and K3PO4, with dimethyl sulfoxide (DMSO) as the solvent.
Under these conditions, the team obtained the desired defluoroalkylation products in mostly moderate to excellent yields. The team proposes a reaction mechanism that involves the formation of a quinuclidinyl N-radical cation, which reacts with an alkoxide zinc intermediate formed from the alcohol in a hydrogen atom transfer (HAT) reaction to give an alkyl radical. This radical combines with an alkenyl radical that is formed via fluoride elimination from the gem-difluoroalkene. Overall, the work allows the visible-light-promoted defluorinative alkylation of gem-difluoroalkenes with alcohols as the alkyl source under mild conditions.
- Defluoroalkylation of gem-Difluoroalkenes with Alcohols via C–F/C–H Coupling,
Congjian Xia, Haiyang Hu, Wengang Xu, Baokai Yang, Qi Shao, Mingbo Wu,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03982


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
