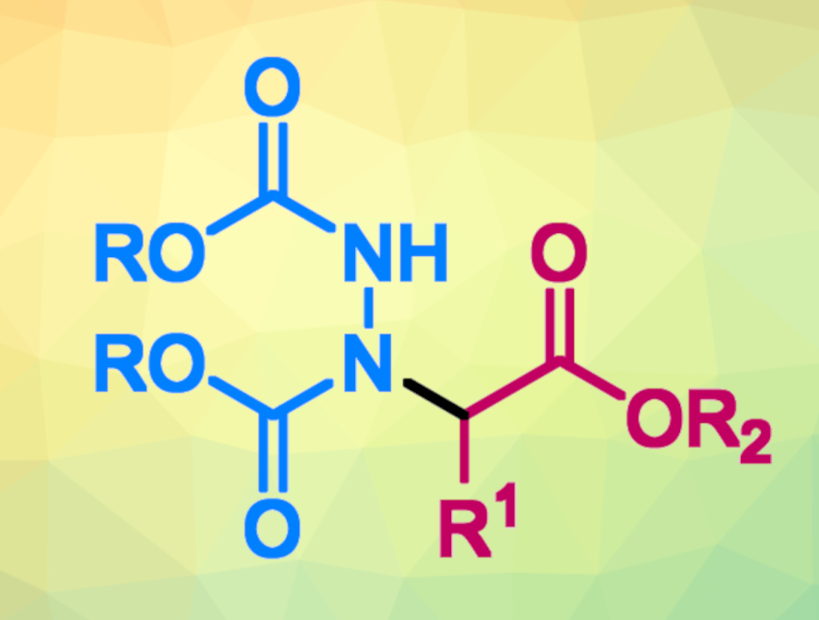
Dialkyl azodicarboxylates can serve as useful nitrogen sources in organic synthesis. For example, they can be used for the electrophilic amination of enolizable compounds, forming a new carbon–nitrogen bond. In this context, decarboxylative processes involving a β-ketocarboxylic acid as a precursor of the nucleophile are generally limited to cyclic compounds.
Erwan Le Gall, Marc Presset, Université Paris-Est Créteil, Thiais, France, and colleagues have developed a method for the decarboxylative amination of substituted malonic acids half oxyesters (SMAHOs) via reactions with dialkyl azodicarboxylates. This type of reaction provides access to α-aminoester derivatives and could be useful, e.g., to obtain unnatural α-amino acids. The reactions between various azodicarboxylates and SMAHOs (pictured below) were promoted by 1,4-diazabicyclo[2.2.2]octane (DABCO) as an organocatalyst and performed in toluene at 80 °C.
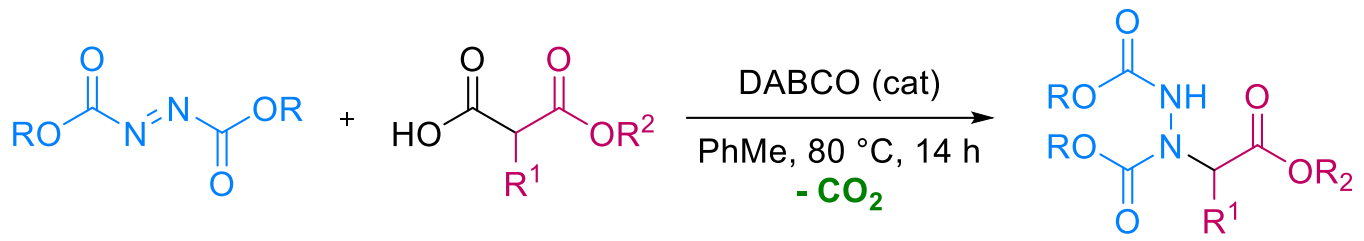
The desired coupling products (pictured) were obtained in mostly moderate to excellent yields, and the reaction can be performed under mild conditions. The work gives an example of the utility of SMAHOs as nucleophiles in coupling reactions leading to nitrogen-containing compounds.
- Decarboxylative Amination of SMAHOs by Dialkyl Azodicarboxylates,
Marine Pinaud, Erwan Le Gall, Marc Presset,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400128