Many natural products and pharmaceutically active compounds contain piperidine units. One way to synthesize piperidine derivatives is the dearomatization of pyridines. Usually, this type of reaction involves a hydrogenation of the pyridines or the addition of nucleophiles to activated pyridines, and many existing dearomatization methods are limited to the introduction of hydrogen atoms or substituents connected via carbon atoms. The introduction of heteroatom substituents is less well-explored.
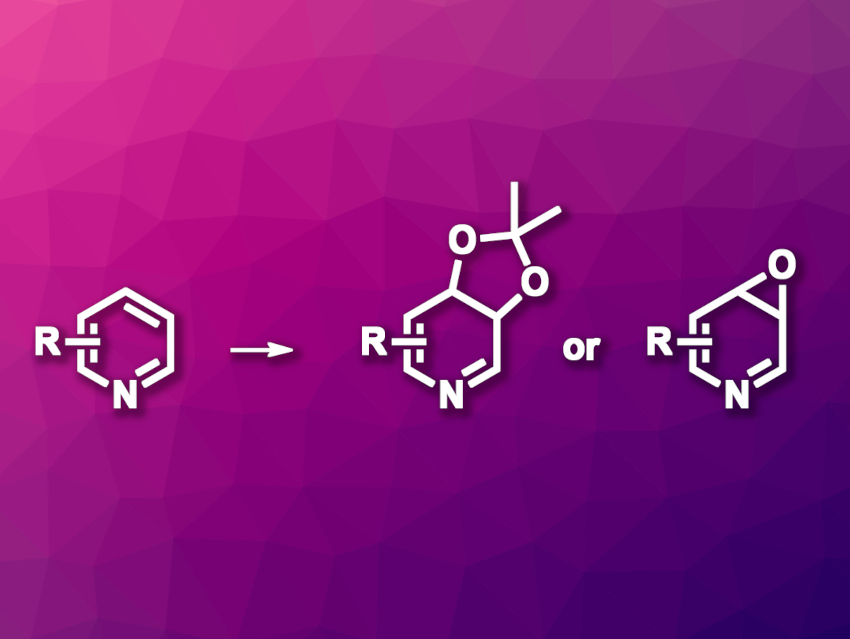
David Sarlah, University of Illinois, Urbana, USA, and colleagues have developed an approach to the oxidative dearomatization of pyridines, mediated by arenophiles and leading to dihydropyridine cis-diols and epoxides (general reaction pictured). The team used N-methyl-1,2,4-triazoline-3,5-dione (MTAD) as an arenophile, which undergoes cycloadditions with pyridines. Combining this reaction with olefin oxidations then gives the desired heteroatom-substituted piperidines.
The team reacted different substituted pyridines with MTAD in a photocyclization in acetone under visible light, followed by an oxidation using osmium tetroxide and a cycloreversion to remove the MTAD unit and obtain the desired dihydropyridine cis-diols. Alternatively, the MTAD-based cycloadducts can be used to synthesize epoxides with manganese(II) perchlorate, followed by cycloreversion to remove the MTAD. The resulting epoxides are in equilibrium with the corresponding seven-membered 1,4-oxazepines, which the team used to synthesize oxazepine derivatives. Overall, the work provides a path to useful functionalized heterocycles from pyridines without substrate preactivation.
- Oxidative Dearomatization of Pyridines,
Zohaib Siddiqi, Tanner W. Bingham, Tsukasa Shimakawa, Kevin D. Hesp, Andre Shavnya, David Sarlah,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c13603