The article looks at the personal and leadership qualities of David Milstein on the occasion of the 75th birthday of this well-known pioneering organometallic and homogeneous catalysis chemist.
The photo above shows the Prof. David Milstein Street in Rehovot, Israel, located in close proximity to the Weizmann Institute of Science. The caption reads “Born 1947. Israeli chemist, Professor at the Weizmann Institute in the fields of chemistry and physics [sic], Israel Prize Laureate.”
Introduction
Professor David Milstein (see Fig. 1) is a well-known pioneering academic in the areas of organometallic chemistry and homogeneous catalysis. He is credited with making great strides in the activation of strong covalent bonds using transition-metal complexes of so-called pincer ligands, inventing a family of catalysts based on these ligands (aptly named Milstein catalysts), discovering fundamentally new concepts in catalysis, and developing new catalytic transformations. All of these have been used by the Milstein group, as well as many others throughout the world, to develop new kinds of chemistry, including sustainable catalysis.

Figure 1. David Milstein.
Throughout his career, David and his group have made several seminal discoveries involving bond activation and catalysis, which have become milestones in various fields of research, ranging from fundamental organometallic chemistry to surface chemistry. As a leading academic, David has profoundly influenced generations of Ph.D. students and postdoctoral researchers in his lab, many of whom are now independent academics and scientists in the chemical industry. His 75th birthday is a befitting ceremonial occasion to highlight his achievements and contributions in his first five decades of research in organometallic chemistry and homogeneous catalysis.
Early Life, Education, and Training
From Germany to Israel
David was born in 1947 in Ulm, Germany, in the same hospital where Albert Einstein was born seven decades earlier. The Milstein family had taken refuge there after World War II, living in a displaced persons camp, and moved to the newly founded State of Israel in 1949. There, David would grow up in Rehovot, a town roughly 20 km south of Tel Aviv, where the Weizmann Institute of Science is located.
After graduating from high school, David pursued his passion for chemistry by enrolling at the Hebrew University of Jerusalem. During his time as a student, he met Adi, and they got married in the summer of 1971.
Stille Coupling Reaction
David received his bachelor’s degree in 1968, and his master’s degree in 1969, both with distinction. He then went on to pursue his Ph.D. degree in organic chemistry under the supervision of Professor Jochanan Blum, graduating summa cum laude in 1976. This was the beginning of his life-long relationship with transition-metal complexes.
Immediately after completing his graduate studies, David moved with his family to Colorado, USA, to join the group of Professor J. K. Stille as a postdoctoral researcher. During this period, he developed, together with Professor Stille, the landmark Stille coupling reaction for selective Pd-catalyzed cross-coupling transformations in organic synthesis (see Scheme 1), an area of research that would later gain recognition in the form of the Nobel Prize in Chemistry for 2010 [1,2]. Notably, most of the seminal papers on the Stille coupling reaction are coauthored by David and his former postdoctoral advisor. It has been noted on multiple occasions that Stille could have shared the 2010 Nobel Prize in Chemistry had he not died in an airplane accident in 1989 [3].
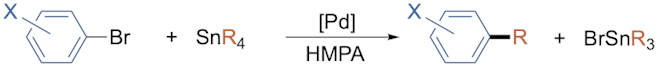 Scheme 1. Example of the Stille coupling reaction from David’s postdoctoral research
Scheme 1. Example of the Stille coupling reaction from David’s postdoctoral research
([Pd] = [(Ph3P)2Pd(CH2Ph)Cl]; X = CH3, OCH3, F, CH3C(O); R = alkyl, aryl).
A Career in Industry
In 1979, after completing his postdoctoral training with Stille, David moved to an industrial position at DuPont’s Central Research and Development Department (CR&D) in Wilmington, DE, USA, where he first worked as a Senior Research Chemist and then as a Group Leader in the catalysis field. This department was an inspiring environment and home for other well-known chemists, who later went on to develop highly successful independent academic careers, such as Richard R. Schrock (Nobel Laureate, 2005).
Fundamental Chemical Transformations in Homogeneous Catalysis
Spending time at DuPont had a profound effect on David’s thinking as a chemist. As part of his work there, he was involved in mechanistic investigations of fundamental chemical transformations in homogeneous catalysis, primarily based on rhodium and iridium complexes, which are relevant to basic processes in the chemical industry [4-6]. Such reactions have a significant economic and environmental impact since they constitute the very beginning of the value-added chains of bulk chemical production.
This work resulted in 20 articles in peer-reviewed journals, as well as two patents, and included, among other things, the activation of small molecules by electron-rich complexes. For example, David described what was, at the time, a very rare case of oxidative addition of water to an Ir(I) center, leading to a thermally stable mononuclear cishydrido-hydroxo complex (see Scheme 2a) [5].
N–H Bond Activations
David and his group were also the first to report the oxidative addition of ammonia to a mononuclear late-transition-metal complex (see Scheme 2b) [7]. This eventually led to the discovery of the first olefin hydroamination process catalyzed by a transition-metal complex (see Scheme 2c) [8].
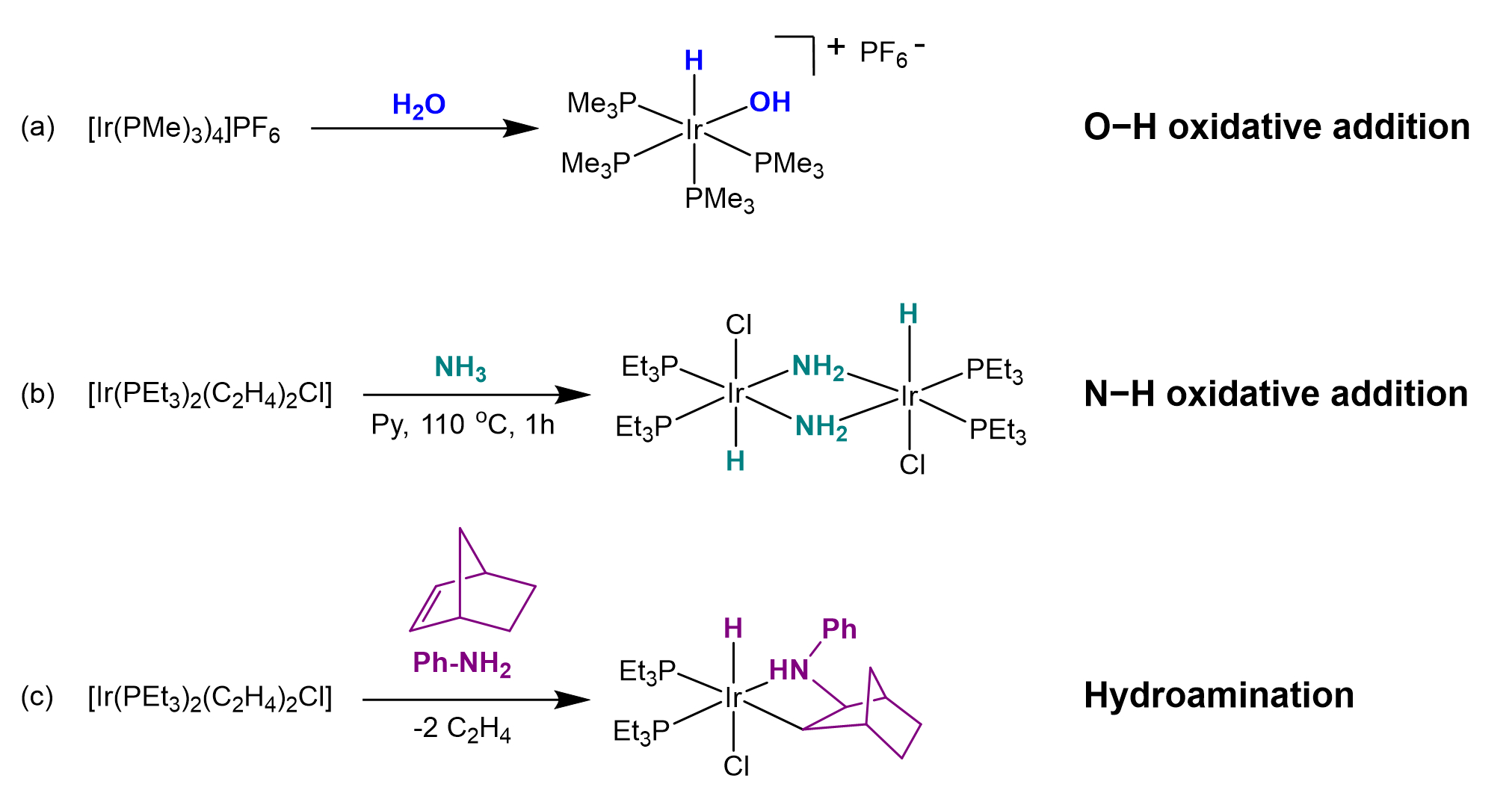
Scheme 2. Select reactions from articles published by David during his time at DuPont.
This fruitful period of time at DuPont appears to have had a profound effect on David’s subsequent academic career, and is well-reflected in his work, especially in the more recent development of fundamental environmentally benign catalytic transformations.
Independent Career at the Weizmann Institute of Science
After eight years at DuPont Central Research, David returned to Israel in 1987 to begin his independent career as an Associate Professor at the Department of Organic Chemistry, Weizmann Institute of Science, Rehovot. In 1993, he was promoted to Full Professor and later served as head of the department for three consecutive terms, from 1996 until 2005. In 2000, David founded the Kimmel Center for Molecular Design, which he directed until 2017.
Pioneering New Pincer Ligand Chemistry
Soon after joining the Weizmann Institute, David and his group began exploring the chemistry of transition-metal complexes of pincer-type ligands. These ligands, so named because of their distinctive structures and ability to strongly bond metal atoms and ions using their two “side-arms” (see Scheme 3), have been known since the 1970s. However, the Milstein group would use such ligands in unprecedented ways, and also introduce many previously unknown pincer ligands, initially employed for the study of fundamental organometallic and inorganic reactions and structures, and then for the construction of various catalysts. With time, these ligands would become a defining characteristic of David’s wide-ranging research.
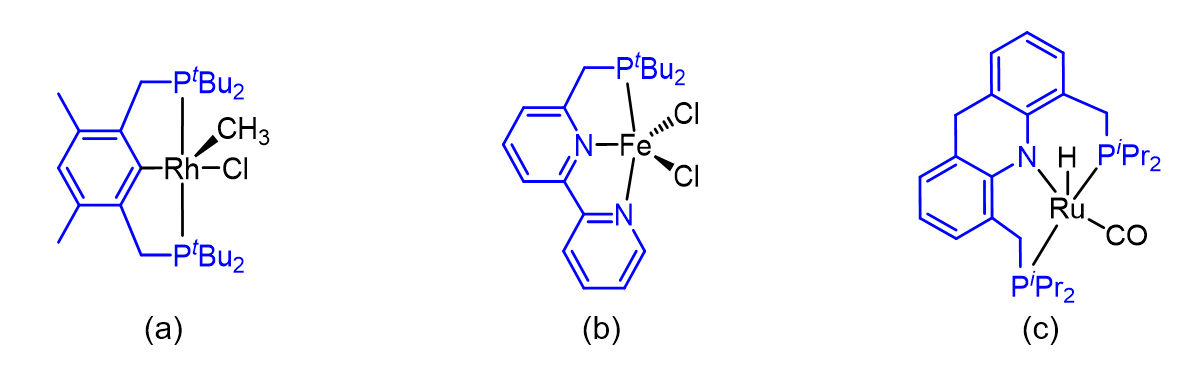
Scheme 3. Examples of pincer ligands reported by the Milstein group, in coordination with various metal centers: (a) Rh(III) complex of an anionic PCP-type ligand; (b) Fe(II) complex of a neutral PNN-type ligand; (c) Ru(II) complex of an anionic PNP-type ligand. In each case, the pincer ligand framework is highlighted in blue.
It is customary to classify pincer ligands according to the identity of the atoms that are directly bonded to the metal center, and their relative position within the ligand. For example, the pincer ligand shown in Scheme 3a is coordinated to the Rh(III) center through two phosphorus atoms from the side-arms and a central carbon atom, and is therefore classified as a PCP-type ligand. Correspondingly, the pincer ligands shown in Schemes 3b and 3c are denoted PNN and PNP.
During the first two decades, work in the Milstein group focused primarily on stoichiometric metal-mediated activation of strong covalent bonds and related reactions, leading to many important discoveries and providing crucial mechanistic insights into industrially important processes. A few notable examples include the first reported cleavage of an unactivated C−C bond by insertion of a metal center in solution, involving a rhodium complex (see Scheme 4a) [9]; an unprecedented catalytic activation of C−F bonds for the defluorination of polyfluorobenzenes, also promoted in solution by rhodium complexes (see Scheme 4b) [10]; water splitting by a combination of thermal H2 evolution and photochemical O2 generation, mediated by ruthenium complexes (see Scheme 4c) [11]; and the isolation and characterization of a rare terminal Pt(IV)-oxo complex, which reacts as both an oxygen donor and an electrophile (see Scheme 4d) [12].
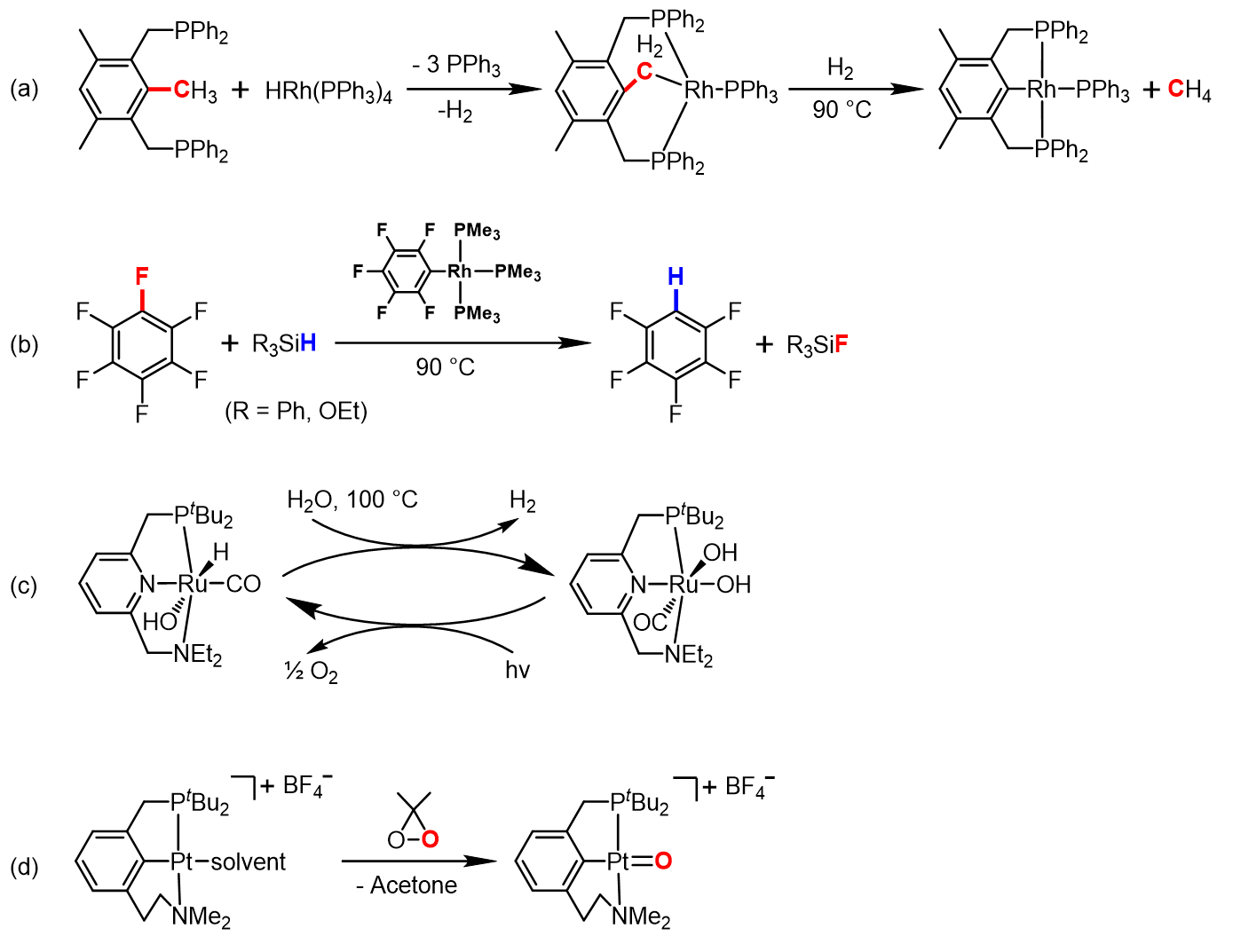
Scheme 4. Notable examples of the pioneering work of the Milstein group involving strong covalent bond activation and related reactions: (a) Rh-mediated C−C bond cleavage in solution; (b) catalytic C−F bond activation with an Rh complex; (c) water splitting using Ru complexes; (d) isolation of a rare terminal Pt(IV)-oxo complex.
During the 2000s, David and his group made significant discoveries in catalysis, which would lead the Milstein group to shift its focus from fundamental questions in inorganic and organometallic chemistry to the design of new catalysts and new kinds of catalysis, with a special emphasis on sustainable processes. First and foremost, the group would pioneer the utilization of so-called metal-ligand cooperation in pincer complexes, typically containing transition metals.
Metal-Ligand Cooperation Using Pincer Ligand Platforms
Reactions involving transition-metal complexes, including those used as catalysts, typically occur at the metal center itself, while the ligands around it do not directly participate in the process, other than dissociating from the metal center to make room for other incoming ligands, such as a catalytic substrate. Common processes that take place at the metal center include oxidative addition and reductive elimination, during which the metal atom or ion changes its oxidation state.
By contrast, in biological systems, there are many metal-containing enzymes that can activate covalent bonds through intimate cooperation between the metal center and its ligands, resulting in the chemical modification of both. This mode of reactivity in natural catalytic systems, which is known as metal-ligand cooperation (MLC), has inspired researchers to adopt similar approaches in the design of synthetic metal-based catalysts [13,14].
A significant development in this field came in 2005, when David and his group reported a previously-unknown kind of MLC involving dearomatization/rearomatization cycles in a ruthenium complex of a pyridine-based PNP-type pincer ligand, with no associated changes in the oxidation state of the metal center (see Scheme 5a) [15]. This MLC enabled the complex to reversibly split H2 between the metal and pincer ligand, and, more importantly, dehydrogenate alcohols and catalyze their coupling into esters with accompanying liberation of H2 as the only byproduct. This type of catalytic reaction, known as acceptorless dehydrogenative coupling, would soon become a characteristic feature of research in the Milstein group (see below).
The arsenal of pincer complexes capable of MLC would grow with time, as David and his group went on to explore other pincer ligands and metal centers. For example, in 2006, they reported a PNP-Ir(I) complex able to activate the strong C−H bonds of benzene (see Scheme 5b) [16], and in 2010, they reported an acridine-based PNP-Ru(II) complex capable of splitting H2 by long-range MLC (see Scheme 5c) [17]. They have also demonstrated the occurrence of MLC in pincer complexes of base metals, such as Mn, Fe, Ni, and Co [18], and even with main group elements like Zn [19] and B [20].
The scope of substrates affected by these complexes has also expanded significantly, as they were shown to activate other covalent bonds, such as O−H, N−H, and B−H. The unique catalytic activity of these pincer complexes attracted increasing attention throughout the academic world, as well as the chemical industry. In fact, the pyridine-based PNN-Ru and acridine-based PNP-Ru complexes presented in Scheme 5, which were shown to catalyze industrially-important transformations involving alcohols, have been made commercially available. They are known as the Milstein Catalyst and the Milstein Acridine Catalyst, respectively.
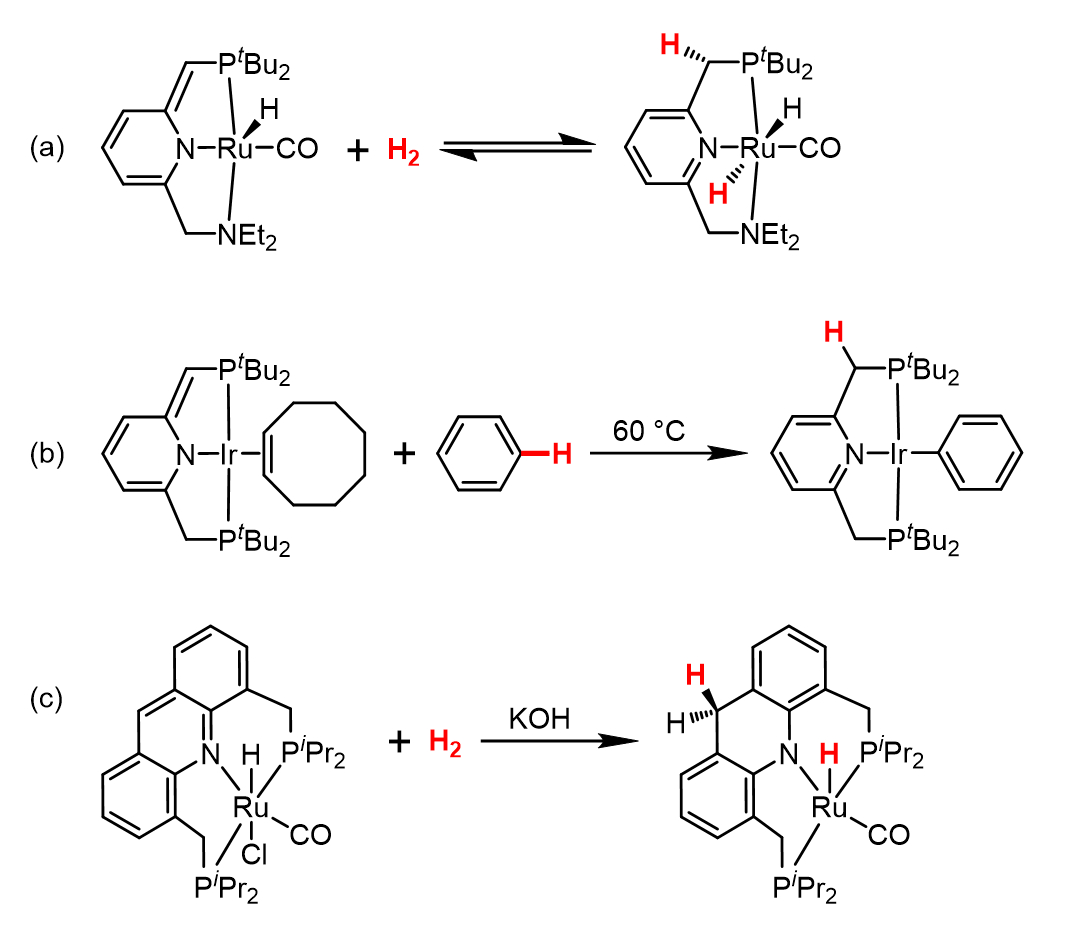
Scheme 5. Examples of metal-ligand cooperation via dearomatization/rearomatization discovered by the Milstein group: H2 splitting by a dearomatized pyridine-based PNN-Ru(II) complex; (b) C−H activation by a dearomatized pyridine-based PNP-Ir(I) complex; (c) H2 splitting by an aromatic acridine-based PNP-Ru(II) complex.
The Milstein Catalysts in Energy- and Sustainability-Related Applications
New Sustainable Processes Involving Acceptorless Dehydrogenation and Hydrogenation Reactions for the Synthesis of Organic Compounds
The Milstein catalysts and their various analogs have been used by David and his group for the efficient synthesis of an array of organic compounds, several examples of which are presented in Scheme 6, as well as in recent review articles [13,14]. These processes involved either acceptorless dehydrogenation or hydrogenation, making them both atom-economical and sustainable.
Among these reactions, a major breakthrough was the first example of amide synthesis through dehydrogenative coupling of alcohols and amines, catalyzed by the PNN-Ru complex I (see Scheme 6) [21]. This discovery was selected by the journal Science as one of the top ten breakthroughs of the year 2007. Analogous ruthenium pincer catalysts have been utilized for the high-yielding synthesis of amide-containing pharmaceuticals, namely, moclobemide, itopride, and trimethobenzamide, as recently reported by the Milstein group [22].
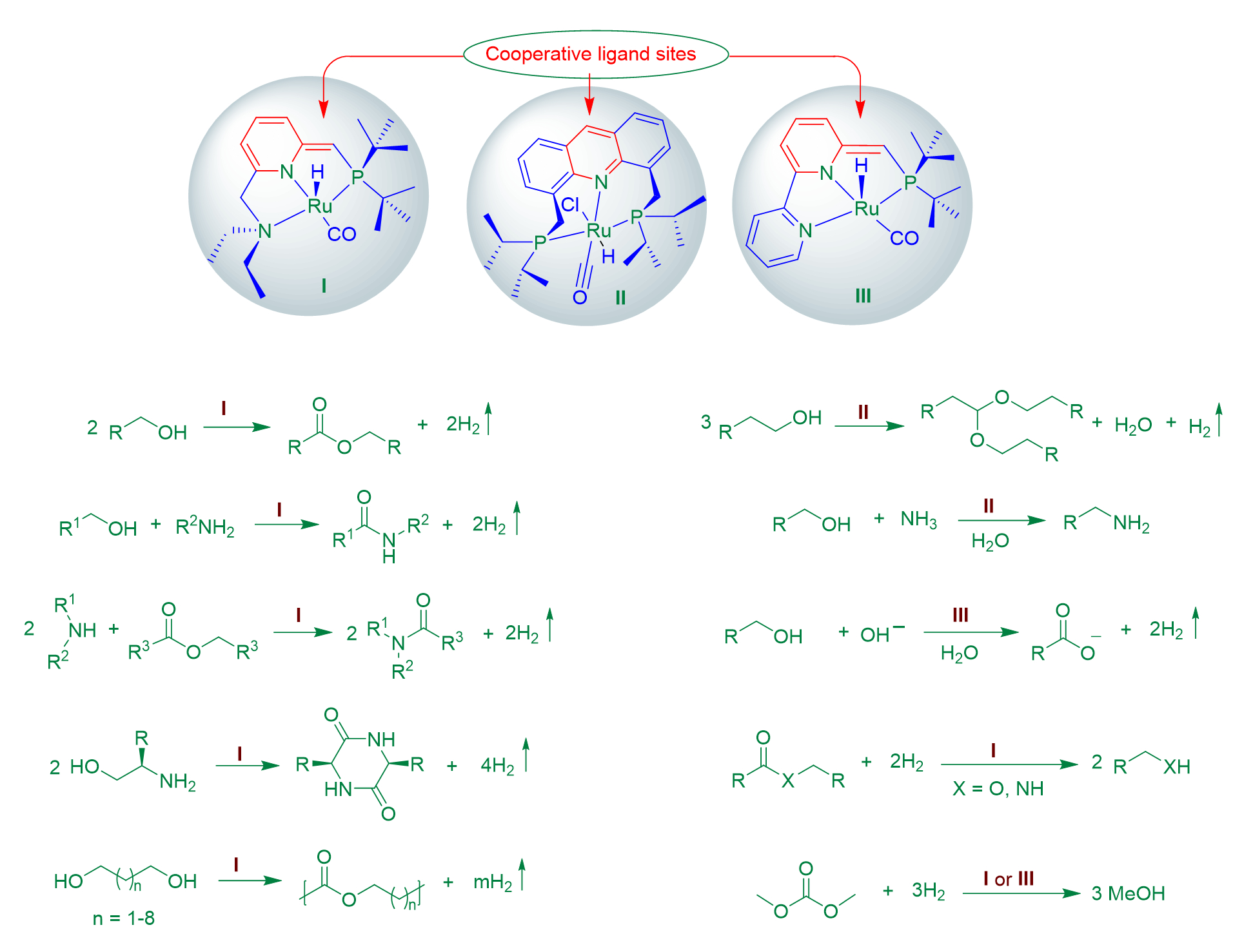
Scheme 6. Examples of catalytic reactions involving acceptorless dehydrogenation and hydrogenation developed by the Milstein group. Catalyst structures are shown at the top.
New Catalysts Using Earth-Abundant Metals and the Concept of Template Catalysis Using Metal-Ligand Cooperation (MLC)
A crucial feature of bond activation by MLC in the Milstein catalysts is that it does not involve changes in the oxidation state of the metal center through oxidative addition, reductive elimination, or redox processes. This has prompted David and his group to explore other metal centers beyond precious metals like Ru [23], leading to the development of pincer complexes of widely-available base metals, such as Fe, Mn, Ni, and Co.
The Milstein group, as well as other groups throughout the world, have shown that such complexes can perform dehydrogenative and hydrogenative catalysis, some of which could previously be achieved only with precious metals [24,25]. For instance, in 2016, David and his group reported the first example of a manganese catalyst for a dehydrogenative transformation, in the form of a PNP-Mn(I) pincer complex that catalyzes the synthesis of imines by acceptorless dehydrogenative coupling of alcohols and amines [26].
Using this and other Mn complexes, the Milstein group also introduced a new catalytic approach called “template catalysis”, where the catalytic transformation is mostly performed by the ligand framework, whereas the role of the metal center is primarily structural [27,28]. This approach has been used to catalyze Michael-type reactions under mild conditions.
Development of Hydrogen Storage Materials and Water Splitting
David and his group have made notable contributions to the development of sustainable energy systems by introducing new catalysts for the storage and release of H2 gas using various liquid organic hydrogen carriers (LOHCs). Recently, they reported the dehydrogenation of neat formic acid into H2 and CO2 by an acridine-based PNP-Ru(II) complex (see Scheme 7a), which maintained its catalytic activity for 50 days and achieved a total turnover number of over 1.7 million [29].
Prior to this, they developed fundamentally new and potentially renewable LOHC systems based on the reversible dehydrogenative coupling of alcohols and amines, namely, ethylenediamine and ethanol (see Scheme 7b) [30], ethylenediamine and 1,4-butanediol (see Scheme 7c) [31], 2-aminoethanol (see Scheme 7d) [32], and ethylene glycol (see Scheme 7e) [33], employing various PNN- and PNP-Ru(II) catalysts. Detailed information on these systems, and the application of such catalysts toward the development of hydrogen and methanol economies, can be found in a recent review article by David and coworkers [34]. In related work, the Milstein group has also been able to demonstrate stoichiometric splitting of water by a PNN-Ru(II) complex (see Scheme 4c), as mentioned above [11].
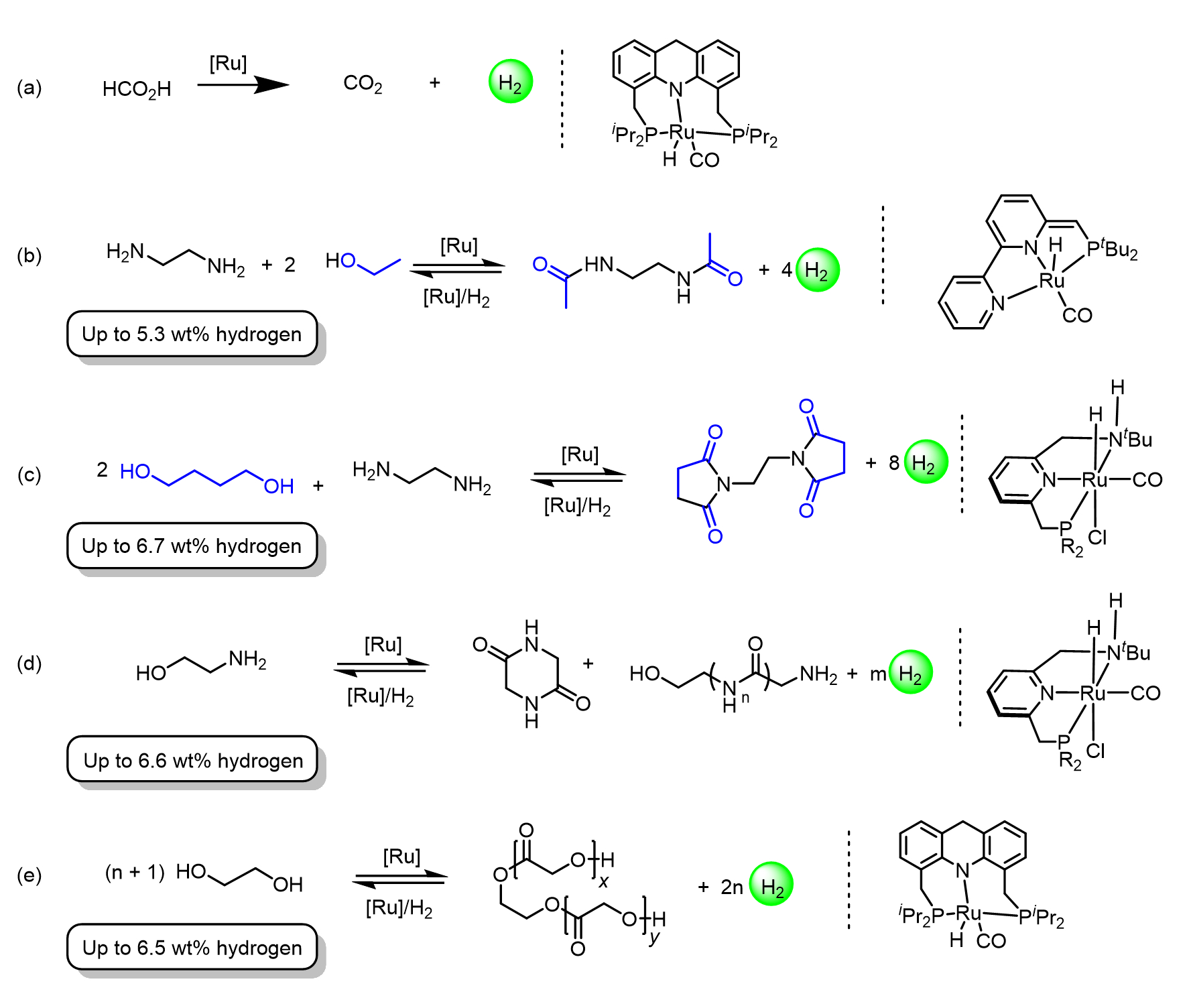
Scheme 7. Liquid organic hydrogen carrier systems developed by the Milstein group, based on ruthenium pincer complexes as catalysts.
Conversion of Greenhouse Gases into Valuable Chemicals
The Milstein group has also made advances in sustainability-related chemistry by showing that the greenhouse gases CO2 and N2O can be converted into benign and even industrially-useful chemicals. David and his group demonstrated a unique mode of CO2 activation via metal-ligand cooperation, as demonstrated in Scheme 8a [35].
Using CO2 as a C1 synthon was demonstrated by indirect hydrogenation of CO2 into methanol via catalytic hydrogenation of formates, carbonates, and carbamates, compounds that can be readily synthesized from CO2 (see Scheme 8b) [34]. Furthermore, the Milstein group has also reported the reduction of N2O using H2, CO, and silanes, catalyzed by ruthenium-pincer complexes (see Scheme 8c) [36–38].
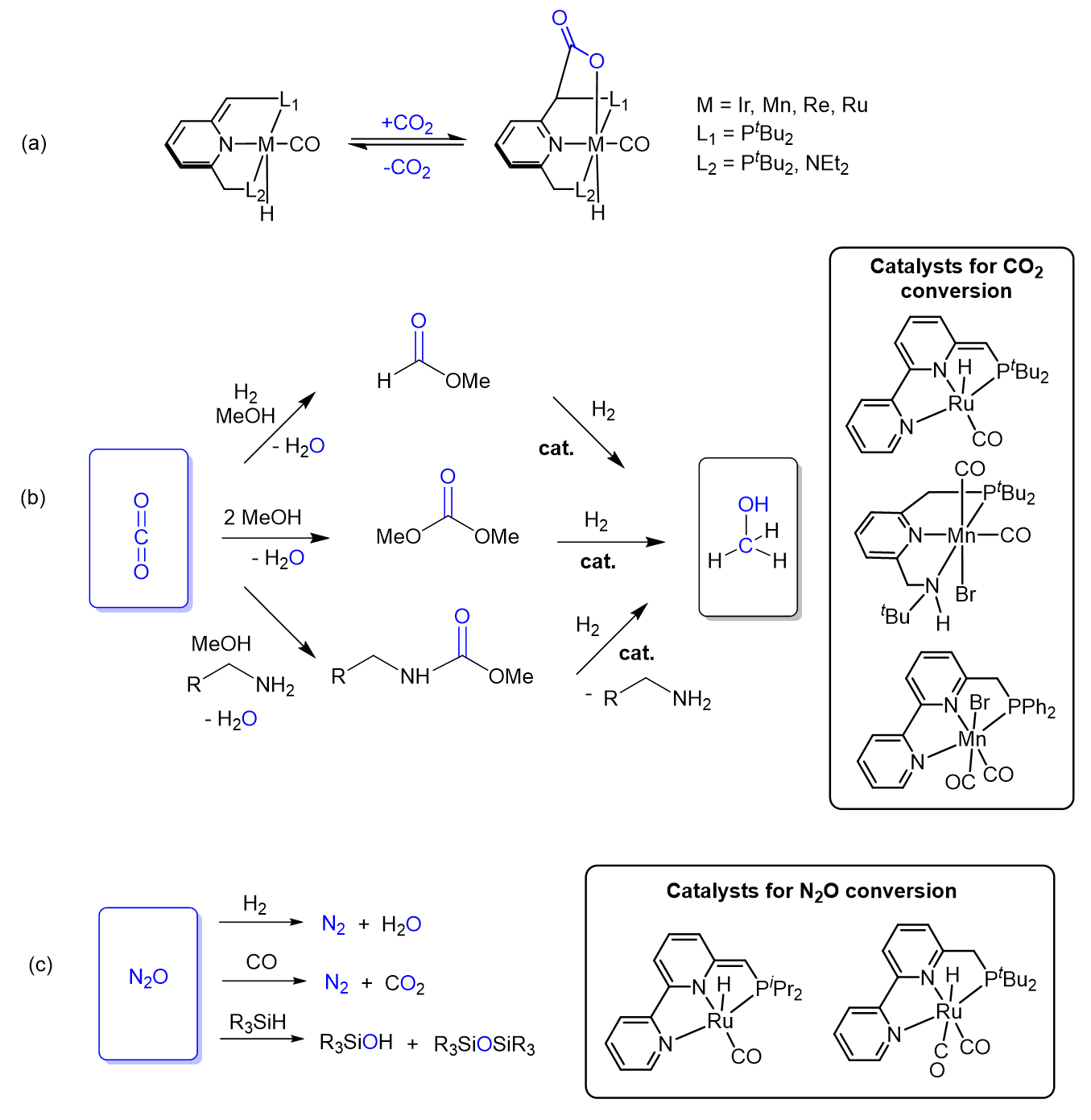
Scheme 8. Conversion of the greenhouse gases CO2 and N2O into benign products promoted by pincer complexes developed by the Milstein group: (a) Reversible CO2 activation by MLC; (b) indirect CO2 hydrogenation into methanol; (c) reduction of N2O.
Biofuel Production from Ethanol
Using an acridine-based PNP-Ru complex (see Scheme 6, catalyst II), the Milstein group has developed a catalytic system for the conversion of ethanol, a renewable feedstock material, into biofuel comprised of butanol and higher alcohols [39]. This system exhibited a turnover number of more than 18,000 in 7 days, which was a record achievement in 2016, when this work was reported. More details on the production of fuels from biomass can be found in a recent review article by David and coworkers [34].
Hydrogenative Depolymerization of Plastic Waste for a Circular Economy
The development of new technologies for the closed-loop production and recycling of plastics is an important challenge of current times. Along this line, the Milstein catalysts and their analogs have been utilized for the synthesis and depolymerization of various types of plastics. For example, in 2012, Robertson and coworkers utilized these catalysts for the synthesis of polyesters by dehydrogenative coupling of diols [40]. In 2014, the same group reported the reverse reaction, i.e., hydrogenative depolymerization of polyesters into diols, promoted by the Milstein catalysts [41].
Similarly, these catalysts were used to demonstrate the first example of nylon synthesis by the dehydrogenative coupling of diols and diamines, as reported independently by the Guan [42] and Milstein [43] groups in 2011 and 2012, respectively. The reverse reaction, i.e., hydrogenative depolymerization of nylons into diols and diamines, was recently reported independently by the Milstein [44] and Schaub [45] groups, who used analogs of the Milstein catalysts.
Analogs of these catalysts have also been utilized for the hydrogenative depolymerization of polycarbonates and polyurethanes [36]. Scheme 9 presents examples of hydrogenative depolymerization of various plastics using the Milstein catalysts or their analogs.
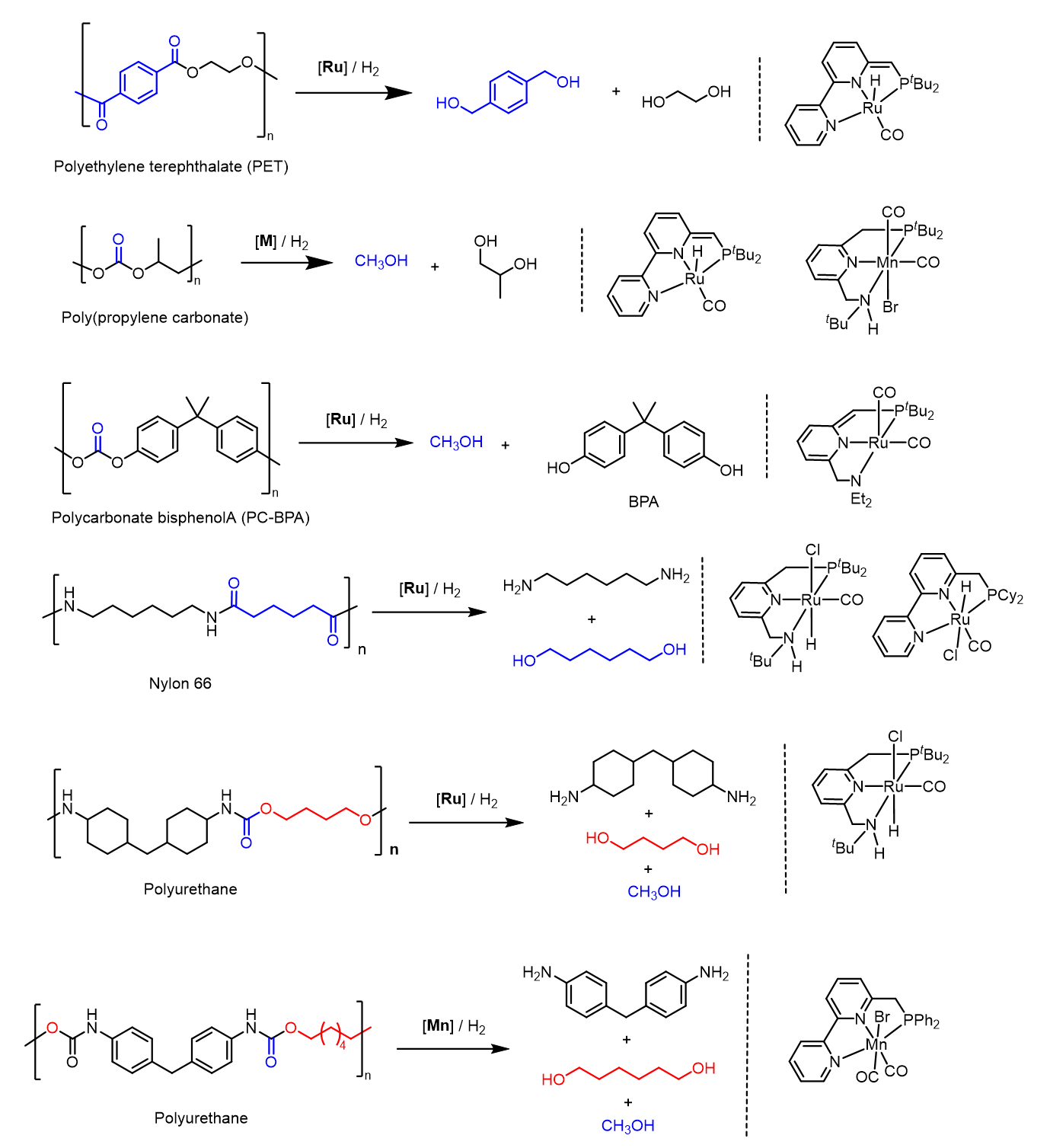
Scheme 9. Hydrogenative depolymerization of various plastics using the Milstein catalysts or their analogs.
The Person David Milstein
Research Philosophy
There are several aspects to David’s research philosophy. While discussing research outcomes in personal or group meetings, he often mentions that “time is precious, and one should use it wisely”. David has always focused on solving challenging problems by inventing new reactions, catalysts, or processes to positively impact the academic world, the chemical industry, and the environment. Another related aspect is novelty in research, which can be clearly seen in many of his publications. David has been strongly motivated to delve into new research areas, based on new concepts and reactions.
David as a Principal Investigator and Supervisor
David is a highly devoted scientist who is excited by the scientific endeavor. Even now, as he reaches the age of 75, he still seems as energetic as a young faculty member at the beginning of his career. At the same time, as can be attested by his current and former group members, David is a patient and approachable supervisor, who does not impose his views on group members and does not micromanage them. As he likes to say, group members are “masters of their own time”, and he places great trust in them. He provides them with flexible working hours, the independence to tackle new problems within the realm of the group’s research interests, and the freedom to write a manuscript and reply to reviewer responses.
It can be said that David is a visionary strategist, whereas group members are responsible for the tactics. The academic freedom and independence that he fosters, together with personal encouragement, inquisitive questions, one-on-one meetings, and advice in the right direction, inspire and enable the Milstein group members to explore new frontiers in chemistry, collaborate with one another, and build their confidence as autonomous researchers.
It is noteworthy that the size of David’s group has never exceeded 20 members, and has typically been closer to 10. Taking his reputation into account, this is a relatively small research group. One of his postdocs once asked David why his group was not larger, despite the fact that he would have no problem obtaining the necessary funding. He responded simply: “For sure, it would be no problem for me to get funding for more people, but why? At the end of the day, I would have more papers published, but for the price of losing contact with parts of my group and not being able to take proper care of all of them, as it would simply be too large. That’s not worth a couple of more papers.”
Despite his many achievements in the fields of organometallic chemistry and catalysis, which include several ground-breaking discoveries, David maintains a modest demeanor. Humility is one of his strengths. When a new graduate student or postdoc joins his group, be it from Israel or abroad, David never gives one the feeling that he is “superior”, and treats his group members and colleagues very inclusively, kindly, and respectfully. He is always approachable in person or over email and is open to new ideas, suggestions, and questions. In fact, he has a strict “open door policy”, which means that when he is at the Weizmann Institute, and his office door is open (which is often the case) one could come at any time for a personal discussion.
David also takes great care to give credit when it is due. A representative example is when one of his Ph.D. students made a certain discovery, which was quite significant at the time, but also noted that a former group member had already observed a related result a few years earlier. This former group member was added as a coauthor to the resulting article, even though he was not directly involved in that particular project.
David is supportive of spending time on one’s personal life. Over the years, he used to encourage his foreign group members to explore Israel and would be curious about the places they had discovered. He once told one of his postdocs, “It seems you now know, after one year, more places in Israel than I do.”
Regarding himself, David has often said that he knows three routes from his residence very well: the way to his office, the way to the swimming pool, and the way to the airport. He is a regular visitor to the swimming pool on the Weizmann Institute campus, and on weekends goes for evening walks with his wife Adi. Occasionally, he would also walk into the lab late at night, and would have a casual conversation with group members present at that time. He is also a warm family man, who tremendously enjoys spending time with his grandchildren. Moreover, he considers his group members to be part of his extended family. Throughout the years, many social events have been arranged at his private residence, where David and his wife Adi would generously host group members. As an example of their hospitality, one can mention the occasional instance where they would host vegetarian group members, and Adi would make tremendous efforts to provide them with suitable vegetarian food.
Legacy
Many academics worldwide have been inspired by David and his research. New areas of chemistry have emerged from his work, and many research groups around the world are currently working along these directions. He has already trained several generations of students and postdoctoral researchers, many of whom are now independent academics or industrial scientists, working on organometallic catalysis as well as other fields of chemical research.
Honors and Awards
David has received many honors and awards throughout his career. Among these are the prestigious ACS Organometallics Award (2007), the ENI Prize for Protection of the Environment presented by the President of Italy (2016), the ACS Gabor A. Somorjai Award for Creative Research in Catalysis (2020), and the IUPAC Zhejiang NHU International Award for Advancements in Green Chemistry (2021). On occasion, he would comment that “one should never run after the awards. If one pursues good chemistry, recognition may come on the way. But, when one starts to run for the awards, there is every chance that this person may lose both chemistry and awards.”
In 2012, David received the Israel Prize, which is the highest honor that the State of Israel can bestow on one of its citizens. This prize is given annually to a select group of Israeli academics and public figures, who have been chosen by special committees for their outstanding contributions to the State of Israel. The award ceremony is held on Israeli Independence Day and broadcast on national television. During the ceremony in 2012, David was accompanied by his family and various contemporary and past group members (see Fig. 2).
David is a member of the Israel Academy of Sciences and Humanities, as well as other national academies, namely, the U.S. National Academy of Sciences, the Royal Society, UK, the German National Academy of Sciences Leopoldina, and the European Academy of Sciences.
 Figure 2. Israel prize ceremony, 2012. David holds the prize plaque, accompanied by several of his contemporary and former group members.
Figure 2. Israel prize ceremony, 2012. David holds the prize plaque, accompanied by several of his contemporary and former group members.
We wish David continued success in both his professional and personal life for many years to come.
References
[1] D. Milstein, J. K. Stille, A general, selective, and facile method for ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium, J. Am. Chem. Soc. 1978, 100, 3636–3638. https://doi.org/10.1021/ja00479a077
[2] D. Milstein, J. K. Stille, Palladium-catalyzed coupling of tetraorganotin compounds with aryl and benzyl halides. Synthetic utility and mechanism, J. Am. Chem. Soc. 1979, 101, 4992–4998. https://doi.org/10.1021/ja00511a032
[3] Carbon couplers take the prize, ChemistryWorld 2010.
[4] D. Milstein, The cis-alkyl and cis-acylrhodium and iridium hydrides. Model intermediates in homogeneous catalysis, Acc. Chem. Res. 1984, 17, 221–226. https://doi.org/10.1021/ar00102a004
[5] D. Milstein, J. C. Calabrese, I. D. Williams, Formation, structures, and reactivity of cis-hydroxy-, cis-methoxy-, and cis-mercaptoiridium hydrides. Oxidative addition of water to Ir(I), J. Am. Chem. Soc. 1986, 108, 6387–6389. https://doi.org/10.1021/ja00280a045
[6] D. Milstein, W. C. Fultz, J. C. Calabrese, Hydroxyacetyliridium and -rhodium complexes: model compounds for carbonyl hydrogenation, J. Am. Chem. Soc. 1986, 108, 1336–1338. ttps://doi.org/10.1021/ja00266a056
[7] A. L. Casalnuovo, J. C. Calabrese, D. Milstein, Nitrogen-hydrogen activation. 1. Oxidative addition of ammonia to iridium(I). Isolation, structural characterization and reactivity of amidoiridium hydrides, Inorg. Chem. 1987, 26, 971–973. https://doi.org/10.1021/ic00254a001
[8] A. L. Casalnuovo, J. C. Calabrese, D. Milstein, Rational design in homogeneous catalysis. Iridium(I)-catalyzed addition of aniline to norbornylene via nitrogen-hydrogen activation, J. Am. Chem. Soc. 1988, 110, 6738–6744. https://doi.org/10.1021/ja00228a022
[9] M. Gozin, A. Weisman, Y. Ben-David, D. Milstein, Activation of a carbon–carbon bond in solution by transition-metal insertion, Nature 1993, 364, 699–701. https://doi.org/10.1038/364699a0
[10] M. Aizenberg, D. Milstein, Catalytic activation of carbon-fluorine bonds by a soluble transition metal complex, Science 1994, 265, 359-361. https://doi.org/10.1126/science.265.5170.359
[11] S. W. Kohl, L. Weiner, L. Schwartsburd, L. Konstantinovski, L. J. W. Shimon, Y. Ben David, M. A. Iron, D. Milstein, Consecutive Thermal H2 and Light-Induced O2 Evolution from Water Promoted by a Metal Complex, Science 2009, 324, 74–77. https://doi.org/10.1126/science.1168600
[12] E. Poverenov, I. Efremenko, A. I. Frenkel, Y. Ben-David, L. J. W. Shimon, G. Leitus, L. Konstantinovski, J. M. L. Martin, D. Milstein, Evidence for a terminal Pt(iv)-oxo complex exhibiting diverse reactivity, Nature 2008, 455, 1093–1096. https://doi.org/10.1038/nature07356
[13] T. Shimbayashi, K. Fujita, Recent Advances in Homogeneous Catalysis via Metal–Ligand Cooperation Involving Aromatization and Dearomatization, Catalysis 2020, 10(6), 635. https://doi.org/10.3390/catal10060635
[14] J. R. Khusnutdinova, D. Milstein, Metal–Ligand Cooperation, Angew. Chem. Int. Ed. 2015, 54, 12236–12273. https://doi.org/10.1002/anie.201503873
[15] J. Zhang, G. Leitus, Y. Ben-David, D. Milstein, Facile Conversion of Alcohols into Esters and Dihydrogen Catalyzed by New Ruthenium Complexes, J. Am. Chem. Soc. 2005, 127, 10840–10841. https://doi.org/10.1021/ja052862b
[16] E. Ben-Ari, G. Leitus, L. J. W. Shimon, D. Milstein, Metal−Ligand Cooperation in C−H and H2 Activation by an Electron-Rich PNP Ir(I) System: Facile Ligand Dearomatization−Aromatization as Key Steps, J. Am. Chem. Soc. 2006, 128, 15390–15391. https://doi.org/10.1021/ja066411i
[17] C. Gunanathan, B. Gnanaprakasam, M. A. Iron, L. J. W. Shimon, D. Milstein, “Long-Range” Metal−Ligand Cooperation in H2 Activation and Ammonia-Promoted Hydride Transfer with a Ruthenium−Acridine Pincer Complex, J. Am. Chem. Soc. 2010, 132, 14763–14765. https://doi.org/10.1021/ja107770y
[18] A. Mukherjee, D. Milstein, Homogeneous Catalysis by Cobalt and Manganese Pincer Complexes, ACS Catal. 2018, 8, 11435–11469. https://doi.org/10.1021/acscatal.8b02869
[19] M. Rauch, S. Kar, A. Kumar, L. Avram, L. J. W. Shimon, D. Milstein, Metal–Ligand Cooperation Facilitates Bond Activation and Catalytic Hydrogenation with Zinc Pincer Complexes, J. Am. Chem. Soc. 2020, 142, 14513–14521. https://doi.org/10.1021/jacs.0c05500
[20] U. Gellrich, Y. Diskin-Posner, L. J. W. Shimon, D. Milstein, Reversible Aromaticity Transfer in a Bora-Cycle: Boron–Ligand Cooperation, J. Am. Chem. Soc. 2016, 138, 13307–13313. https://doi.org/10.1021/jacs.6b07454
[21] C. Gunanathan, Y. Ben-David, D. Milstein, Direct Synthesis of Amides from Alcohols and Amines with Liberation of H2, Science 2007, 317, 790–792. https://doi.org/10.1126/science.1145295
[22] S. Kar, Y. Xie, Q. Q. Zhou, Y. Diskin-Posner, Y. Ben-David, D. Milstein, Near-Ambient-Temperature Dehydrogenative Synthesis of the Amide Bond: Mechanistic Insight and Applications, ACS Catal. 2021, 11, 7383–7393. https://doi.org/10.1021/acscatal.1c00728
[23] D. Milstein, Metal–ligand cooperation by aromatization–dearomatization as a tool in single bond activation, Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140189. https://doi.org/10.1098/rsta.2014.0189
[24] A. Mukherjee, D. Milstein, Homogeneous Catalysis by Cobalt and Manganese Pincer Complexes, ACS Catal. 2018, 8, 11435–11469. https://doi.org/10.1021/acscatal.8b02869
[25] L. Alig, M. Fritz, S. Schneider, First-Row Transition Metal (De)Hydrogenation Catalysis Based On Functional Pincer Ligands, Chem. Rev. 2019, 119, 2681–2751. https://doi.org/10.1021/acs.chemrev.8b00555
[26] A. Mukherjee, A. Nerush, G. Leitus, L. J. W. Shimon, Y. Ben David, N. A. Espinosa Jalapa, D. Milstein, Manganese-Catalyzed Environmentally Benign Dehydrogenative Coupling of Alcohols and Amines to Form Aldimines and H2: A Catalytic and Mechanistic Study, J. Am. Chem. Soc. 2016, 138, 4298–4301. https://doi.org/10.1021/jacs.5b13519
[27] S. Tang, D. Milstein, Template catalysis by manganese pincer complexes: oxa- and aza-Michael additions to unsaturated nitriles, Chem. Sci. 2019, 10, 8990–8994. https://doi.org/10.1039/C9SC03269J
[28] A. Nerush, M. Vogt, U. Gellrich, G. Leitus, Y. Ben-David, D. Milstein, Template Catalysis by Metal–Ligand Cooperation. C–C Bond Formation via Conjugate Addition of Non-activated Nitriles under Mild, Base-free Conditions Catalyzed by a Manganese Pincer Complex, J. Am. Chem. Soc. 2016, 138, 6985–6997. https://doi.org/10.1021/jacs.5b13208
[29] S. Kar, M. Rauch, G. Leitus, Y. Ben-David, D. Milstein, Highly efficient additive-free dehydrogenation of neat formic acid, Nat. Catal. 2021, 4, 193–201. https://doi.org/10.1038/s41929-021-00575-4
[30] P. Hu, Y. Ben-David, D. Milstein, Rechargeable Hydrogen Storage System Based on the Dehydrogenative Coupling of Ethylenediamine with Ethanol, Angew. Chem. Int. Ed. 2016, 55, 1061–1064. https://doi.org/10.1002/anie.201505704
[31] A. Kumar, T. Janes, N. A. Espinosa-Jalapa, D. Milstein, Selective Hydrogenation of Cyclic Imides to Diols and Amines and Its Application in the Development of a Liquid Organic Hydrogen Carrier, J. Am. Chem. Soc. 2018, 140, 7453–7457. https://doi.org/10.1021/jacs.8b04581
[32] P. Hu, E. Fogler, Y. Diskin-Posner, M. A. Iron, D. Milstein, A novel liquid organic hydrogen carrier system based on catalytic peptide formation and hydrogenation, Nat. Commun. 2015, 6, 6859. https://doi.org/10.1038/ncomms7859
[33] Y. Q. Zou, N. von Wolff, A. Anaby, Y. Xie, D. Milstein, Ethylene glycol as an efficient and reversible liquid-organic hydrogen carrier, Nat. Catal. 2019, 2, 415–422. https://doi.org/10.1038/s41929-019-0265-z
[34] A. Kumar, P. Daw, D. Milstein, Homogeneous Catalysis for Sustainable Energy: Hydrogen and Methanol Economies, Fuels from Biomass, and Related Topics, Chem. Rev. 2022, 122, 385–441. https://doi.org/10.1021/acs.chemrev.1c00412
[35] A. Kumar, D. Milstein, in Metal-Ligand Co-operativity, Catalysis and the Pincer-Metal Platform (Eds: Gerard van Koten, Karl Kirchner, Marc-Etienne Moret), Springer Cham, Switzerland, 2021, pp. 1–23. ISBN: 978-3-030-68915-5
[36] A. Kumar, C. Gao, Homogeneous (De)hydrogenative Catalysis for Circular Chemistry – Using Waste as a Resource, ChemCatChem 2021, 13, 1105–1134. https://doi.org/10.1002/cctc.202001404
[37] R. Zeng, M. Feller, Y. Ben-David, D. Milstein, Hydrogenation and Hydrosilylation of Nitrous Oxide Homogeneously Catalyzed by a Metal Complex, J. Am. Chem. Soc. 2017, 139, 5720–5723. https://doi.org/10.1021/jacs.7b02124
[38] R. Zeng, M. Feller, Y. Diskin-Posner, L. J. W. Shimon, Y. Ben-David, D. Milstein, CO Oxidation by N2O Homogeneously Catalyzed by Ruthenium Hydride Pincer Complexes Indicating a New Mechanism, J. Am. Chem. Soc. 2018, 140, 7061–7064. https://doi.org/10.1021/jacs.8b03927
[39] Y. Xie, Y. Ben-David, L. J. W. Shimon, D. Milstein, Highly Efficient Process for Production of Biofuel from Ethanol Catalyzed by Ruthenium Pincer Complexes, J. Am. Chem. Soc. 2016, 138, 9077–9080. https://doi.org/10.1021/jacs.6b05433
[40] D. M. Hunsicker, B. C. Dauphinais, S. P. Mc Ilrath, N. J. Robertson, Synthesis of High Molecular Weight Polyesters via In Vacuo Dehydrogenation Polymerization of Diols, Macromol. Rapid Commun. 2012, 33, 232–236. https://doi.org/10.1002/marc.201100653
[41] E. M. Krall, T. W. Klein, R. J. Andersen, A. J. Nett, R. W. Glasgow, D. S. Reader, B. C. Dauphinais, S. P. Mc Ilrath, A. A. Fischer, M. J. Carney, D. J. Hudson, N. J. Robertson, Controlled hydrogenative depolymerization of polyesters and polycarbonates catalyzed by ruthenium(ii) PNN pincer complexes, Chem. Commun. 2014, 50, 4884–4887. https://doi.org/10.1039/C4CC00541D
[42] H. Zeng, Z. Guan, Direct Synthesis of Polyamides via Catalytic Dehydrogenation of Diols and Diamines, J. Am. Chem. Soc. 2011, 133, 1159–1161. https://doi.org/10.1021/ja106958s
[43] B. Gnanaprakasam, E. Balaraman, C. Gunanathan, D. Milstein, Synthesis of polyamides from diols and diamines with liberation of H2, J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1755–1765. https://doi.org/10.1002/pola.25943
[44] A. Kumar, N. Von Wolff, M. Rauch, Y. Q. Zou, G. Shmul, Y. Ben-David, G. Leitus, L. Avram, D. Milstein, Hydrogenative Depolymerization of Nylons, J. Am. Chem. Soc. 2020, 142, 14267–14275. https://doi.org/10.1021/jacs.0c05675
[45] W. Zhou, P. Neumann, M. Al Batal, F. Rominger, A. S. K. Hashmi, T. Schaub, Depolymerization of Technical-Grade Polyamide 66 and Polyurethane Materials through Hydrogenation, ChemSusChem 2021, 14, 4176. https://doi.org/10.1002/cssc.202002465
Special Collection
To celebrate his 75th birthday, Angewandte Chemie, Chemistry – A European Journal, ChemCatChem, and the European Journal of Inorganic Chemistry have assembled a special collection featuring a selection of articles by Professor David Milstein.
Video Interview
Mario Müller interviewed David Millstein for ChemistryViews: A Good Traveler Has No Fixed Destination




