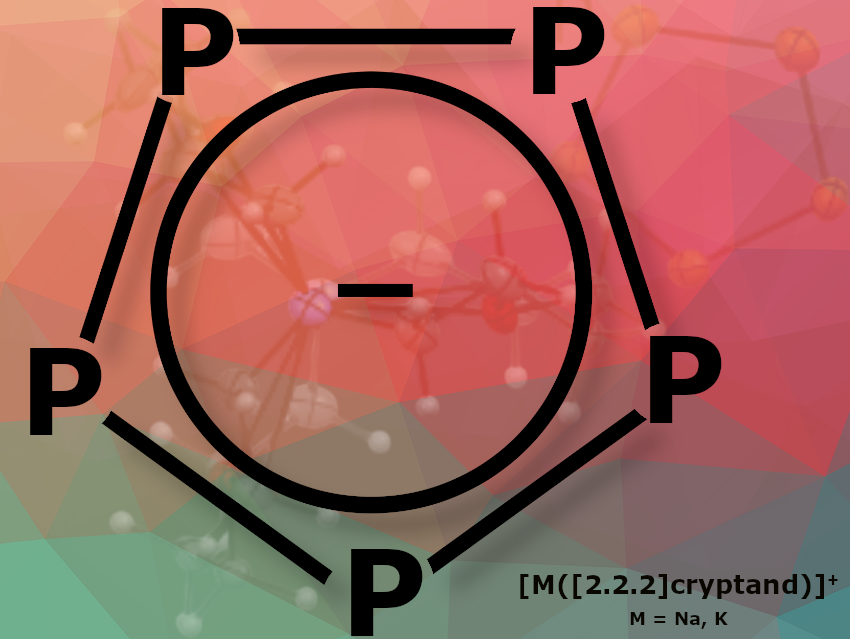
Christian Müller, Freie Universitaet Berlin, Germany, and colleagues have stabilized the cyclo-P₅⁻ anion in the solid state through complexation of the alkaline metal cation with [2.2.2]cryptand, providing the first crystallographic characterization and detailed spectroscopic analysis. The cyclo-P₅⁻ anion has been spectroscopically known since the 1980s, but it was previously prepared and handled only in solution.
The researchers developed two new synthetic routes for alkali metal salts of the cyclopentaphospholide anion. By using [2.2.2]cryptand, they were able to generate the cyclo-P₅⁻ anion from the alkali metal heptaphosphides M₃P₇ (M = Na, K). A similar reactivity was observed when using stoichiometric amounts of white phosphorus, potassium, and [2.2.2]cryptand in THF. The team isolated the salts [M([2.2.2]cryptand)][cyclo-P₅] (M = Na, K). The researchers found that complexing the alkali metal cation with [2.2.2]cryptand made the product surprisingly stable in the solid state. This stability allows the handling of the cyclo-P₅⁻ anion in its uncoordinated form in organic solvents such as THF and CH₂Cl₂, enabling its crystallographic characterization for the first time.
Moreover, the team successfully reacted [Na([2.2.2]cryptand)][cyclo-P₅] with LiCp* and FeCl₂, yielding the ferrocene derivative [Cp*Fe(cyclo-P₅)]. They confirmed the planar D₅h symmetry of cyclo-P₅, both as a ligand in sandwich complexes and in its uncoordinated form.
- Cyclo-P₅⁻ Revisited: The Surprisingly Stable Uncoordinated Pentaphospholide Anion,
Moritz Ernst, Andrey Petrov, Mirjam Schröder, Björn Corzilius, Christian Müller,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202505853