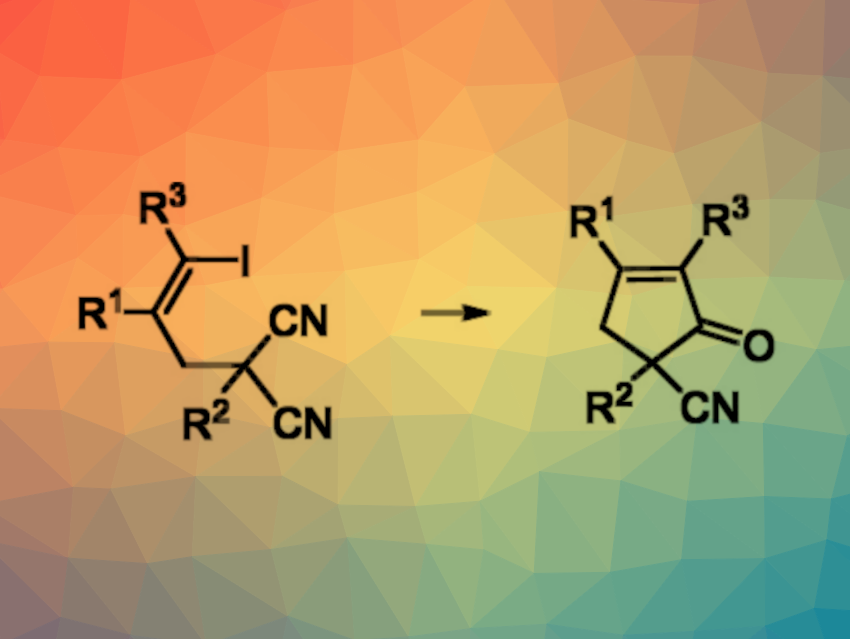
Nitriles can be used as reaction partners in palladium-catalyzed coupling reactions. The insertion of a nitrile into a C–Pd(II) bond can generate an imido-Pd(II) intermediate and release an imine upon protonation.
Xiaofeng Tong, Changzhou University, Jiangsu, China, and Taizhou University, Zhejiang, China, and colleagues have developed a ligand-free Pd(0)-catalyzed cyclization of (Z)-5-iodo-4-pentenenitriles, using HCO2H as a reductant. After imine hydrolysis, this reaction gives cyclopentenone derivatives. The team converted (Z)-5-iodo-4-pentenenitriles with a wide range of different substituents using Pd(OAc)2 as the catalyst, HCO2H as the reductant, N,N-diisopropylethylamine (DIPEA) as a base, and 1,2-dichloroethane (DCE) as the solvent. The reactions were performed at 60 °C. After the reaction was completed, aqueous HCl was added.
The desired cyclopentenone derivatives were obtained in moderate to good yields. The team was also able to directly isolate imine products after the cyclization reaction was completed, which can be particularly useful for products with an acid-sensitive group. According to the researchers, HCO2H acts not only as a reductant but also as a proton source, which is important for the protonation of the imido-Pd(II) intermediate.
- Access to Cyclopentenone via Pd(0)-Catalyzed Reductive Cyclization of (Z)-5-Iodo-4-pentenenitrile,
Ming Dong, Xiaofeng Tong,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03999