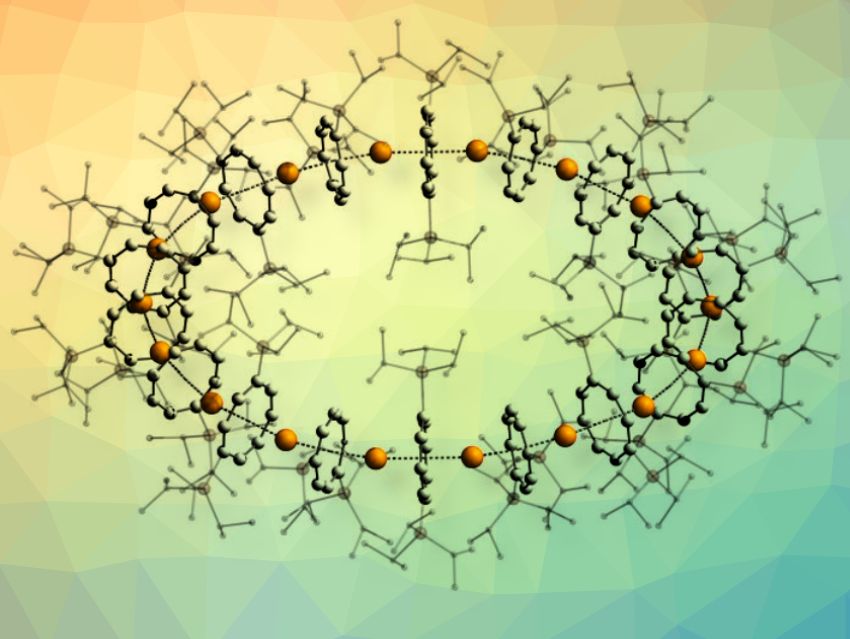
Peter W. Roesky, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have designed, synthesized, and characterized an isomorphous series of nanometer-scale cyclic sandwich complexes (example pictured). In sandwich complexes, a metal ion is π-coordinated by two planar aromatic organic rings. They have been successfully used in a wide variety of applications, including catalysis, electrochemistry, and medicine. However, so far, only linear one-dimensional multidecker sandwich compounds have been investigated.
The research team suggests the term cyclocenes for their circular sandwich compounds. They consist of 18 repeating metallocene sandwich units, forming almost ideally circular, closed rings in the solid state, and are described by the general formula [cyclo-MII(μ-η8:η8-CotTIPS)]18 (M = Sr, Sm, Eu; (Cot = C8H82−) and CotTIPS = 1,4-(iPr3Si)2C8H62−). Cyclocene made with Sm is pictured above. The Cyclocenes are formed from monomers of the metal coordinated with bulky cyclooctatetraene and the solvent tetrahydrofuran (THF) which is then removed.
The pronounced bending is caused by the steric requirements of the Cot substituents. The driving force for the ring formation is the energy gained by ring closure, which is largely influenced by dispersion interactions. The researchers hypothesize that the stability and size of cyclocenes can be tuned by selectively tailoring the ligand systems used. Furthermore, they anticipate that the cyclocene family will open the door for further innovations toward new functional metal-organic materials.
- Synthesis and properties of cyclic sandwich compounds,
Luca Münzfeld, Sebastian Gillhuber, Adrian Hauser, Sergei Lebedkin, Pauline Hädinger, Nicolai D. Knöfel, Christina Zovko, Michael T. Gamer, Florian Weigend, Manfred M. Kappes, Peter W. Roesky
Nature 2023, 620, 92–96.
https://doi.org/10.1038/s41586-023-06192-4