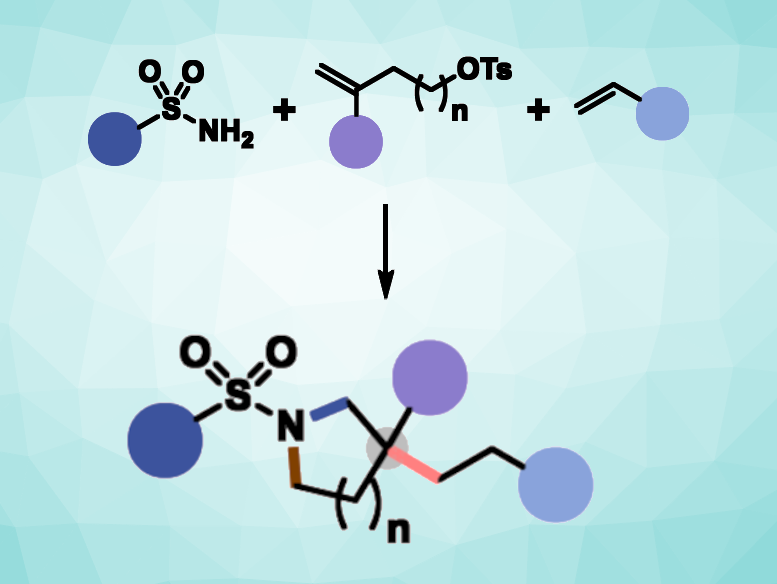
Cyclic amines are important structures found, for example, in natural products, pharmaceutically active compounds, agrochemicals, and organic materials. Thus, effective methods for their synthesis from simple starting materials are in demand. Radical-polar crossover cycloaddition could be a useful tool in this context and can be employed to build cyclic structures in a single step. In a radical-polar crossover mechanism, one intermediate shows radical reactivity and a second intermediate shows polar reactivity. So far, this approach has been used in two-component reactions for the synthesis of cyclic amines.
Huan-Ming Huang, ShanghaiTech University, China, and colleagues have developed a three-component radical-polar crossover cycloaddition for cyclic amine synthesis (general reaction pictured). The approach uses photoredox chemistry to react primary sulfonamides with two different alkenes: an unactivated alkene and an activated alkene/Michael acceptor. The primary sulfonamide acts as a bifunctional reagent, which serves as both a radical precursor and a nucleophile. The three components were reacted in the presence of an iridium-based photocatalyst and K2CO3 as a base, using a PhCF3/tBuOH solvent mixture. The reactions were performed under 450 nm LED light at room temperature.
The desired cyclic amines were obtained in moderate to high yields. The reactions proceed under mild conditions, and the method has a broad functional group tolerance. It allows the construction of β, β-disubstituted cyclic amines with a sterically hindered all-carbon quaternary center, as well as β-monosubstituted cyclic amine derivatives. According to the researchers, the application of primary sulfonamides as bifunctional reagents could also be useful in other transformations.
- Cyclic Amine Synthesis via Catalytic Radical‐Polar Crossover Cycloadditions,
Ying Zhang, Shu-Sheng Chen, Kai-Dian Li, Huan-Ming Huang,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202401671