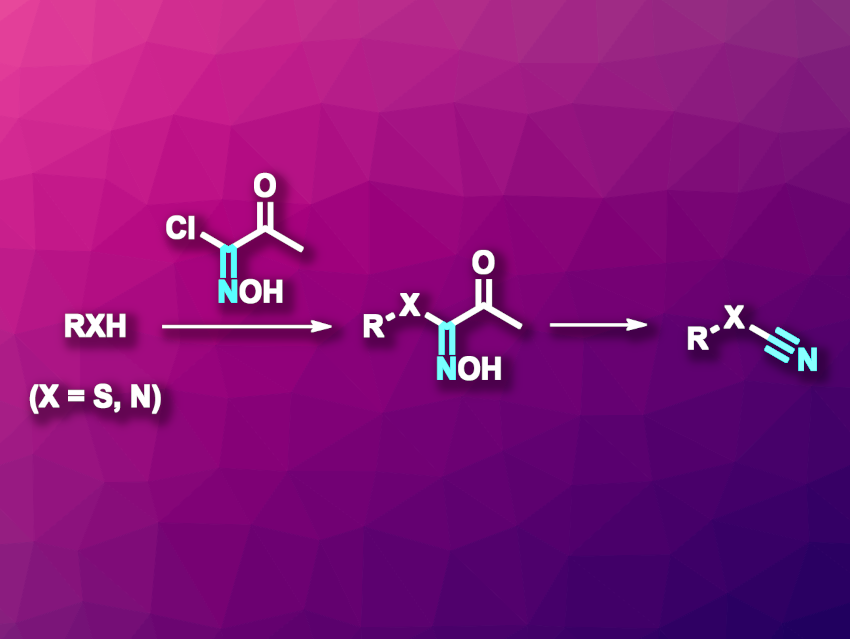
Cyanide (or nitrile) groups in organic compounds can serve as useful sites for further functionalizations. Cyanation reactions can be used to introduce CN groups at C atoms to give nitriles, at S atoms to give thiocyanates, or at N atoms to give cyanamides. The direct use of metal cyanides or cyanogen halides as cyanating agents is an option, but is hampered by their toxicity. Easier-to-handle electrophilic cyanide transfer agents that contain a formal CN+ unit and a leaving group can be useful alternatives.
Hee Nam Lim, Yeungnam University, Gyeongbuk, Republic of Korea, and colleagues have developed a cyanation method for the preparation of thiocyanates and cyanamides (pictured) using N-hydroxy-2-oxopropanimidoyl chloride as a latent cyanide transfer reagent. The transformation involves a two-step, one-pot process. First, the N-hydroxy-2-oxopropanimidoyl chloride reacts with a thiol or an amine, and then a deacetylative C–C bond cleavage releases the thiocyanates or cyanamides. The team performed the first step in tetrahydrofuran (THF) as the solvent in the presence of Et3N as a base, and then added diethylaminosulfur trifluoride (DAST) to induce the deacetylation step. DAST serves as a nucleophilic fluoride donor, which leads to the desired bond cleavage.
Under these conditions, a range of thiols were converted to the corresponding thiocyanates in moderate to good yields. Secondary amines showed reduced yields due to the effects of non-converted educt, so the researchers switched to a two-step process in which the intermediates are first isolated and then undergo the deacetylative cyanation step. This provided access to, e.g., N-cyano heterocycles in mostly moderate to good yields. Using their new method, the team synthesized N-cyanopyrazole from pyrazole for the first time.
- Deacetylative cyanation: a cyanide-free route to thiocyanates and cyanamides,
Si Yeon Kim, Hee Nam Lim,
Chem. Commun. 2024.
https://doi.org/10.1039/D4CC04539D