“Molecular containers”, i.e., cage-like molecules with pores that have sizes in the nanometer range could be useful, e.g., for the adsorption and release of guest molecules or the encapsulation of catalysts. They can be challenging to synthesize, especially when using multiple irreversible reactions, which can give very low yields of the desired molecular container. Dynamic covalent chemistry, in which new covalent bonds are formed in a reversible manner, can be used to solve this problem. However, the resulting products often suffer from insufficient stability under ambient conditions. For example, the covalent bonds in these systems are often formed by condensation reactions, and even trace amounts of water or alcohols can cause bond breaking and decomposition.
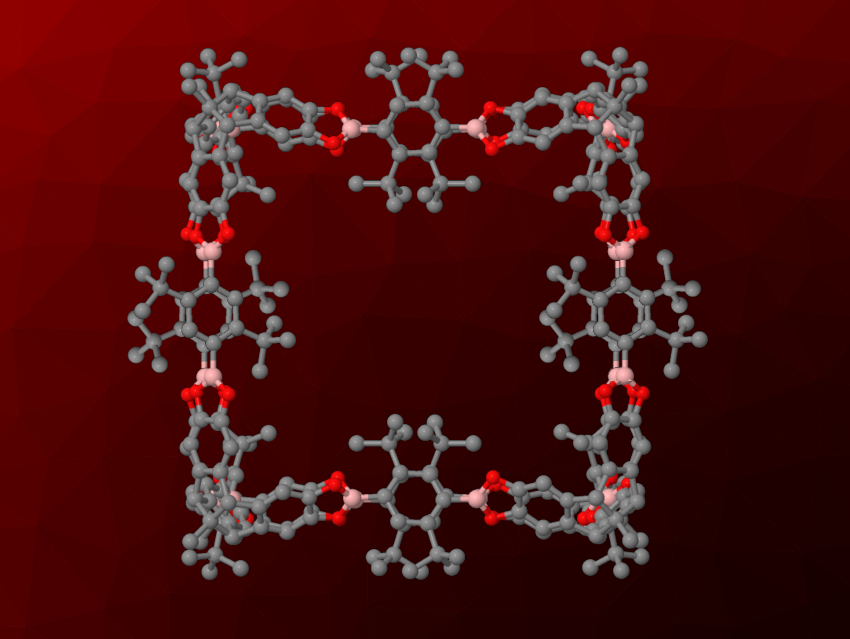
Florian Beuerle, University of Würzburg, Germany, and University of Tübingen, Germany, and colleagues have developed cube-shaped cages connected by boronate esters that show unprecedented stability in the presence of water or alcohols (pictured). The team used bulky tert-butyl groups in the ortho-positions to the boron centers to provide steric shielding and stabilize the bonds holding the cage together. The cages were synthesized from di-tert-butyl-functionalized (1,4-phenylene)diboronic acids (BDBAs), which connect the corners of the cube and form the edges, and hexahydroxy tribenzotriquinacenes (TBTQs), which form the corners of the cube. These building blocks were reacted in the presence of catalytic amounts of AcOH, and the team isolated the desired crystalline cages after 7–10 days.
The cages are formed under catalytic conditions, and they are stable under pH-neutral conditions, even in pure water or MeOH. They can even resist slightly acidic media. “Regular” cages connected by boronate esters without bulky substituents dissolve in these cases. The team showed possible uses of the developed water-stable cages for, e.g., the adsorption of β-carotene, which was released on demand by adding a strongly acidic solution, or the encapsulation of a ruthenium complex, which creates a heterogeneous catalyst for water oxidation.
- A Water-Stable Boronate Ester Cage,
Philipp H. Kirchner, Louis Schramm, Svetlana Ivanova, Kazutaka Shoyama, Frank Würthner, Florian Beuerle,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c12002