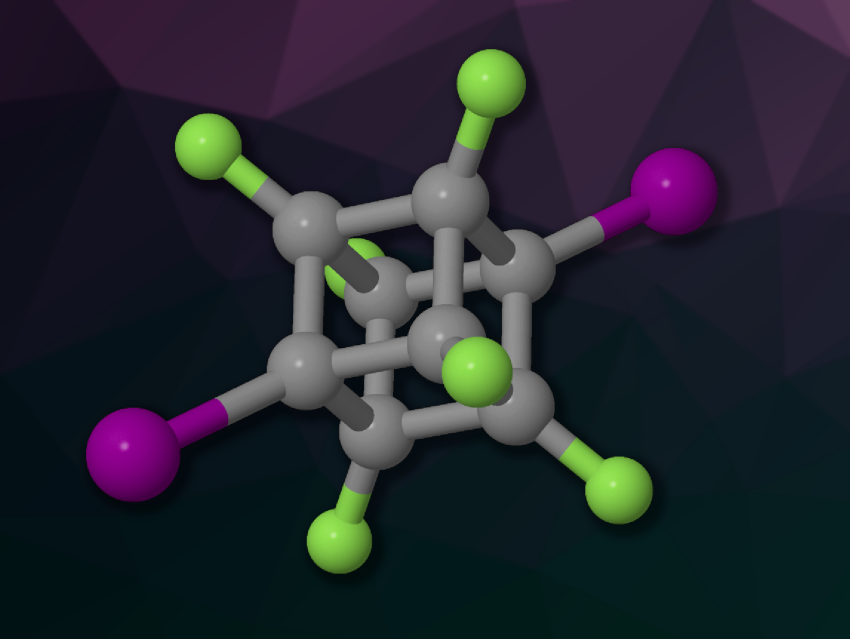
The lengths of chemical bonds can usually provide some information about the nature of the bonds. Large deviations from the average length for a specific type of bond are rare, but there are some known examples of significantly longer or shorter bonds.
Yuta Uetake, Osaka University, Japan, Midori Akiyama, Kyoto University, Japan, and colleagues have studied bonds between tetracoordinated carbon atoms and halogen atoms (Cl, Br, and I), looking for particularly short examples. Halotrinitromethanes (C(NO3)3X, X = Cl, Br, I) are known molecular frameworks that show shortened C(sp3)–halogen bonds. Several factors can contribute to these shortened bonds, including the s-character of the bonding orbital, hyperconjugation, multi-bond character, and Coulombic interactions. These different contributions can be challenging to evaluate separately. The team, thus, looked for another series of compounds with shortened C–X bonds to allow for comparisons, and settled on investigating polyfluorocubanes, which are strained, electron-deficient, molecules with a cube-shaped carbon core.
The researchers synthesized hexafluorodihalocubanes of the type C8F6X2 (example pictured, X = Cl, Br, I) starting from hexafluorocubane, which was deprotonated using lithium bis(trimethylsilyl)amide (LiHMDS) and reacted with aryl sulfonyl halides to introduce the desired halogen atoms. The products were characterized using single-crystal X-ray diffraction, X-ray absorption spectroscopy, and NMR spectroscopy, and also studied using density functional theory (DFT) calculations.
The team found that the C–X bonds of the hexafluorodihalocubanes are shorter than average, and in particular, the iodine-substituted compound features the shortest C(sp3)–I bond reported so far. According to the researchers, the increased s-character at the carbon atom and hyperconjugation are more significant factors for the shortening of the C–X bonds in halotrinitromethanes, while coulombic interactions between the carbon atom and the halogen atom are more significant contributors to bond shortening in the hexafluorodihalocubanes. This effect is dominant for the iodine analogues, leading to the exceptionally short bond in the cubane derivative.
- Exceptionally Short Tetracoordinated Carbon–Halogen Bonds in Hexafluorodihalocubanes,
Masafumi Sugiyama, Yuta Uetake, Nozomu Miyagi, Masaaki Yoshida, Kyoko Nozaki, Takashi Okazoe, Midori Akiyama,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c12732