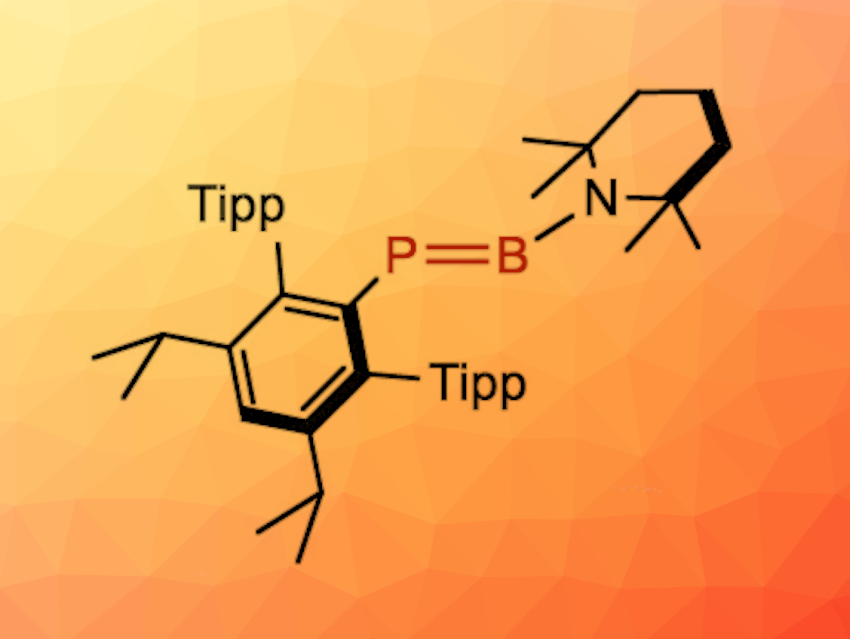
Compounds with multiple bonds between the “heavier” main group elements are interesting research targets. Species with multiple bonds involving elements of group 13 and 15, for example, can be compared with compounds such as olefins or acetylenes. Phosphaborenes (R–P═B–R) are examples of this type of compound.
Ian Manners, University of Victoria, British Columbia, Canada, and colleagues have synthesized a stable monomeric phosphaborene, i.e., Ar*P═B(TMP) (pictured; Ar* = 2,6-bis(triisopropylphenyl)-3,5-diisopropylphenyl, TMP = 2,2,6,6-tetramethylpiperidine). The team started from Ar*Li·Et2O, which was converted to Ar*PH2, followed by deprotonation. A reaction with Br2B(TMP) then led to the formation of a R–P(H)═B(Br)–R-type precursor, and treatment with KHMDS (HMDS = hexamethyldisilazide) gave the desired phosphaborene.
According to the researchers, the compound is the first stable monomeric phosphaborene without stabilization from a coordinated Lewis acid or base or via a “push-pull” strategy. The phosphaborene has the shortest P–B bond length reported so far with 1.741(3) Å. A bond order analysis gave a large Wiberg bond index of ca. 1.97, which is consistent with a double-bond character.
- A Crystalline Monomeric Phosphaborene,
Etienne A. LaPierre, Brian O. Patrick, Ian Manners,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c01942