In living organisms, cells read data from molecular “tapes” (i.e., messenger RNA) and create an output (i.e., proteins). Recreating a similar process with small synthetic molecules is a challenge.
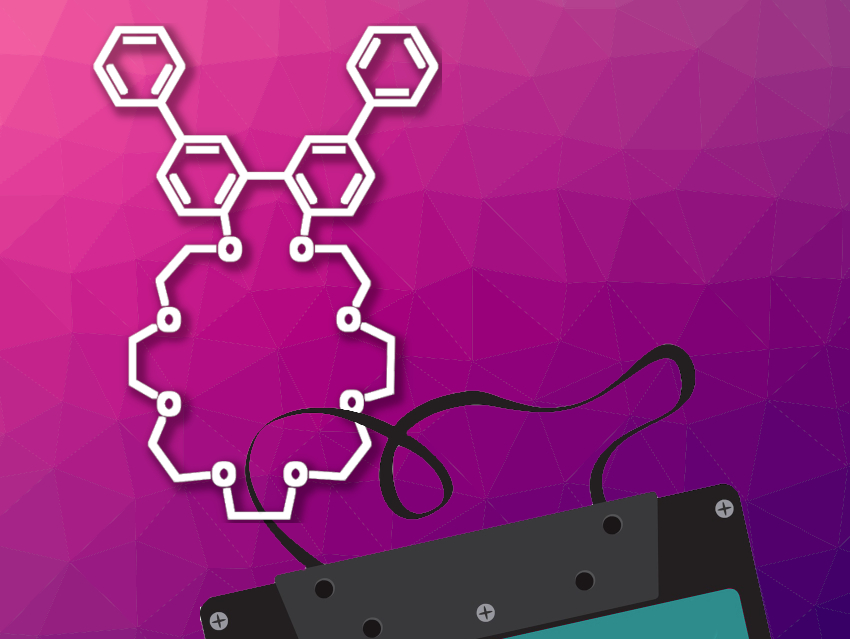
David A. Leigh, University of Manchester, UK, and East China Normal University, Shanghai, China, and colleagues have used a crown ether (pictured) as a synthetic “reading head” that can read out stereochemical information along a strand-like “tape” molecule.
The team chose a crown ether with a 2′,2”-quaterphenyl unit that can have different conformations depending on its interactions with different parts of the molecular tape. The molecular tape features three compartments separated by hydrazone and disulfide barriers. These compartments contain either a protonated asymmetric N-benzyl-α-methylbenzylamine unit or an achiral N-methyltriazolium unit. The barriers along the molecular tape can be “opened” under acidic or basic conditions, respectively. This allows the crown ether “reading head” to move from one compartment of the molecular tape to the next upon pH changes (like a ratchet).
When the crown ether binds to the asymmetric units along the tape, its biaryl group twists and the resulting asymmetry creates a circular dichroism (CD) response. When the crown ether binds to the achiral N-methyltriazolium unit in other parts of the tape, this asymmetry is not induced and there is no CD response. Thus, the team can observe three different signals, which they call 0 for the achiral site and +1 and –1 for the (R)- and (S)-enantiomers of the N-benzyl-α-methylbenzylammonium sites, respectively.
This system allowed the team to read out the sequence of stereochemical information “programmed” into the molecular tape non-destructively. They obtained the information as a string of digits based on the observed CD responses.
- A tape-reading molecular ratchet,
Yansong Ren, Romain Jamagne, Daniel J. Tetlow, David A. Leigh,
Nature 2022.
https://doi.org/10.1038/s41586-022-05305-9