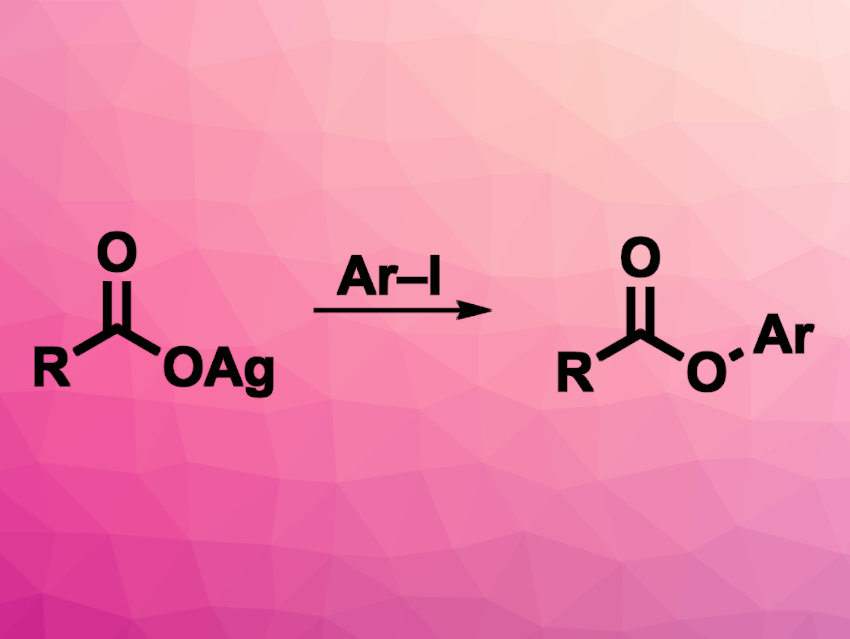
O-Aryl esters are found in many natural products, bioactive compounds, and materials. They can be prepared, e.g., via a transition-metal-catalyzed cross-coupling of aryl halides and carboxylic acid derivatives. However, this type of reaction can have drawbacks because the reductive elimination of RCOO–M–Ar can be sluggish. Gold catalysis with an AuI/AuIII catalytic cycle could help to alleviate this problem.
Bo Xu, Donghua University, Shanghai, China, and colleagues have developed a method for the gold-catalyzed C–O cross-coupling reaction of (hetero)aryl iodides with silver carboxylates. The team used SPhosAuCl (2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl gold(I) chloride) as the catalyst and reacted a range of silver carboxylates with a variety of aryl and heteroaryl iodides in the presence of AgSbF6 and LiNTf2, using HFIP (1,1,1,3,3,3-hexafluoroisopropanol) as a solvent. The reactions were performed at 90 °C.
The team obtained the desired esters in moderate to high yields. The reaction has a broad substrate scope, is insensitive to moisture and air, and can also be used for intramolecular cross-coupling reactions.
- Gold-Catalyzed C–O Cross-Coupling Reactions of Aryl Iodides with Silver Carboxylates,
Guifang Chen, Bo Xu,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02254



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)