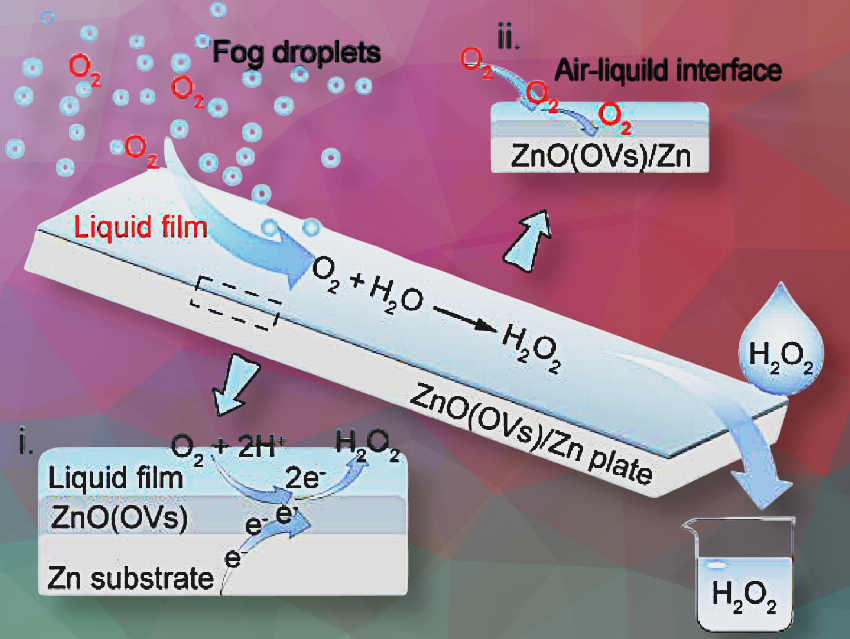
Hydrogen peroxide has a wide range of applications as an eco-friendly and sustainable oxidant. However, the clean, efficient, and convenient synthesis of it remains challenging. Qiangqiang Sun, Southwest Jiaotong University, Chengdu, China, and colleagues have designed an electron-self-supplied catalytic system capable of generating H2O2 from water and atmospheric oxygen without extra energy input.
The catalytic system is made of a ZnO coating containing oxygen vacancies and a Zn substrate (ZnO(OVs)/Zn plate). The Zn substrate provides the necessary electrons while the ZnO(OVs) serves as a catalyst, thus facilitating the reduction of oxygen via a one-step two-electron oxygen reduction reaction (2e– ORR) pathway. The presence of OVs helped to preactivate oxygen and reduce the reaction barrier, thereby promoting the reduction process.
The H2O2 concentration produced by this catalytic system is up to 17.9 mM without any secondary processing. The researchers attribute this remarkably high concentration to the formation of a liquid film on the hydrophilic ZnO surface that promotes the oxygen reduction reaction by faciliating the transfer of oxygen from the ambient air to the catalyst surface.
By integrating with atmospheric fog collection, the researchers say that this system can continuously harvest H2O2 directly from the air. “The ability to collect H2O2 from the air enables this system to provide a potential disinfection solution in remote areas with limited medical and water resources”, the team said.
- A ZnO‐based Catalytic System for the Synthesis of Hydrogen Peroxide from Air,
Lan Wang, Chunyao Fang, Boran Xu, Yunlong Yu, Youmei Liu, Xianbiao Fu, Ang Cao, Qiangqiang Sun, Shaobing Zhou,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202424984