The Sonogashira cross-coupling reaction can be used to form a C–C bond between an organohalide and a terminal alkyne. Acetylene can be used as a coupling partner in this type of reaction for the synthesis of symmetrical diarylethynes. However, acetylene gas can pose safety risks. Calcium carbide can be used as a stable, safe, cost-effective, and convenient alternative alkyne source.
Caixia Xu, Qingqing Bu, Shihezi University, China, and colleagues have developed a direct synthesis of diarylethynes from less-reactive aryl chlorides and calcium carbide (pictured below). The team used a catalyst system composed of Pd(OAc)2, ZnCl2, and SPhos (a phosphine ligand with a biphenyl-based substituent), together with potassium tert-butoxide as a base. Dimethylsulfoxide (DMSO) was used as the solvent, and the reactions were performed at 100 °C.
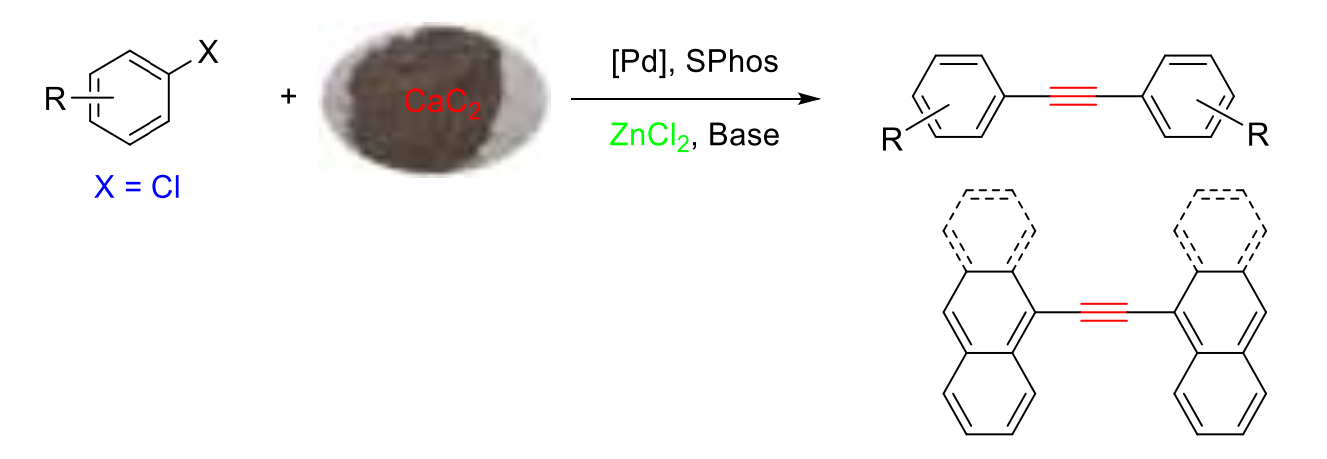
Various symmetrical diarylethynes were obtained in moderate to excellent yields. Aryl chlorides with both electron-donating and electron-withdrawing groups underwent the transformation smoothly, and a variety of functional groups were tolerated. The catalytic system also tolerates challenging fused-ring aromatic substrates such as chloronaphthalene and 9-chloroanthracene.
- Zinc Chloride‐Promoted Coupling Reaction Between Calcium Carbide and Aryl Chloride,
Tianna Jing, Ning Liu, Caixia Xu, Qingqing Bu,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200178