N-Heterocyclic carbenes (NHCs) are often used for the stabilization and surface functionalization of metal nanoparticles (NPs). While monometallic NHC-stabilized NPs, such as gold nanoparticles (AuNPs), are commonly used, NHC-functionalized bimetallic NPs are less well-studied. There are only a few examples, synthesized from free carbenes and metal precursors by either hydrogenation or thermal decomposition.
Ali Nazemi, Université du Québec à Montréal, Canada, and colleagues have used polymerized
NHC complexes with immobilized Au and Ag atoms to obtain bimetallic NPs. The team used poly(1,2,3-triazole)-based polymers as precursors, which were prepared from a diazide-functionalized monomer and an ethylene glycol-based dialkyne-functionalized monomer via a click-reaction polymerization.
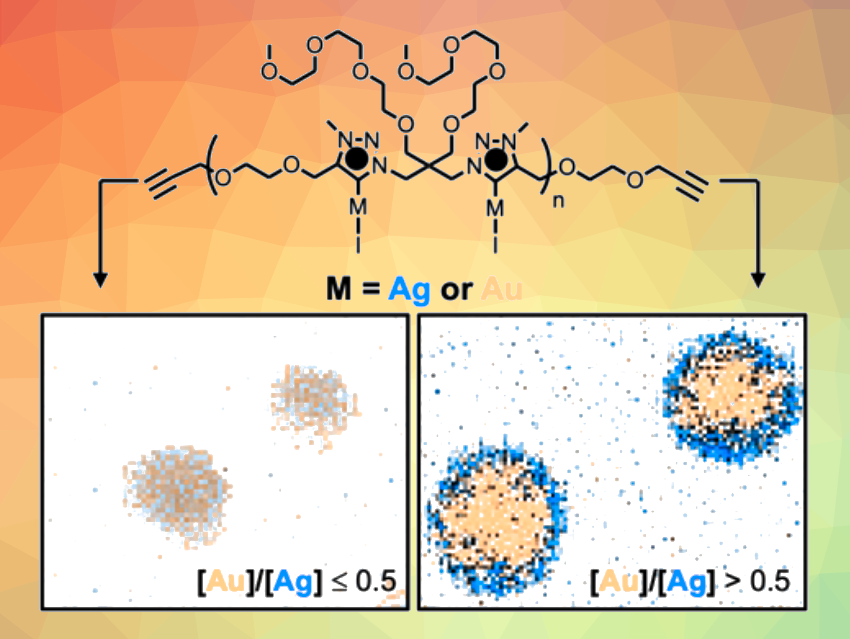
The polymer was quaternized using iodomethane, and silver atoms were introduced using Ag2O. This gives a polymeric mesoionic carbene silver complex. Gold atoms can be introduced into the polymer in controllable ratios by transmetallation using different amounts of (CH3)2SAuCl. A fully gold-functionalized polymer can be obtained by using (CH3)2SAuCl instead of Ag2O in the previous step.
The researchers then converted the synthesized mono- and bimetallic polymers into nanoparticles by reduction with a t-butylamine borane complex. They obtained the corresponding monometallic NPs from the fully Ag- and Au-functionalized polymers. Depending on the ratio of Ag and Au atoms in the mixed-metal polymers, they obtained either alloy-type NPs with a uniform metal distribution or core-shell-type NPs with Au cores and Ag shells (for the polymer with the lowest Ag content).
Overall, the work represents the first path to NHC-protected bimetallic AuAgNPs. The synthetic approach allows for the tuning of the Au/Ag ratio and the formation of either alloy or core-shell NPs. The nanoparticles can be used in an electrocatalytic oxygen reduction reaction.
- Poly(N‐Heterocyclic Carbene)‐Capped Alloy and Core‐Shell AuAg Bimetallic Nanoparticles,
Diep T. H. Nguyen, Samaneh Salek, Lorianne R. Shultz-Johnson, Marilyne Bélanger-Bouliga, Titel Jurca, Joshua C. Byers, Ali Nazemi,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202409800