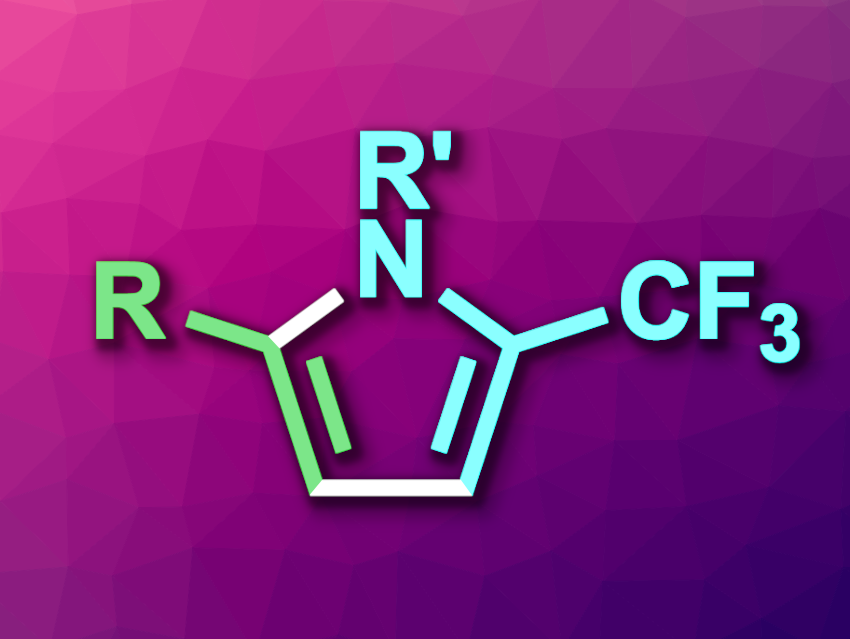
Pyrroles are five-membered heterocycles that have applications, e.g., in drug development or agrochemicals. Trifluoromethyl substituents are often used, e.g., in pharmaceutical chemistry to change the physicochemical properties of compounds. Thus, methods for the synthesis of CF3-functionalized pyrroles are interesting research targets.
Biao Xiong, Nantong University, China, and colleagues have developed a method for the copper-mediated [3+2] cyclization of CF3-imidoyl sulfoxonium ylides and terminal alkynes to give 5-trifluoromethylpyrroles (general product structure pictured). The team used CuOTf as a catalyst and acetonitrile (MeCN) as the solvent to react a wide range of CF3-substituted imidoyl sulfoxonium ylides (F3C–C(=NR’)–CH=SOMe2) with a variety of terminal alkynes. The reactions were performed under a nitrogen atmosphere at 80 °C.
Under these conditions, the desired 5-trifluoromethylpyrroles were obtained in moderate to high yields. The reaction has a broad substrate scope, shows good functional group tolerance, and proceeds under mild conditions. The researchers propose a reaction mechanism that involves copper–carbene-derived radical species. The strategy might also be useful to construct other heterocycles.
- Copper-Mediated Cyclization of Terminal Alkynes with CF3-Imidoyl Sulfoxonium Ylides To Construct 5-Trifluoromethylpyrroles,
E Mi, Li Zhou, Yixin Tong, Xiaodong Qiu, Xiaobao Zeng, Jinlong Li, Biao Xiong,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00423