Ni(II) norcorroles, despite their 16π antiaromaticity, are stable and show unusual reactivity due to their high HOMO and low LUMO. Nucleophiles react with perfect regioselectivity at the distal β-position, whereas electrophiles target the β-positions with regioselectivity that depends on the specific electrophile. The non-aromatic C–C double bonds in the skeleton act like typical alkenes, undergoing hydrogenation or reduction with hydrazine, or [3 + 2] cycloadditions with 1,3-dipoles. Ring-expansion or ring-opening reactions can be induced by activated zwitterionic intermediates, oxidants, and carbenes. Reactions with radical species, however, have not been previously explored.
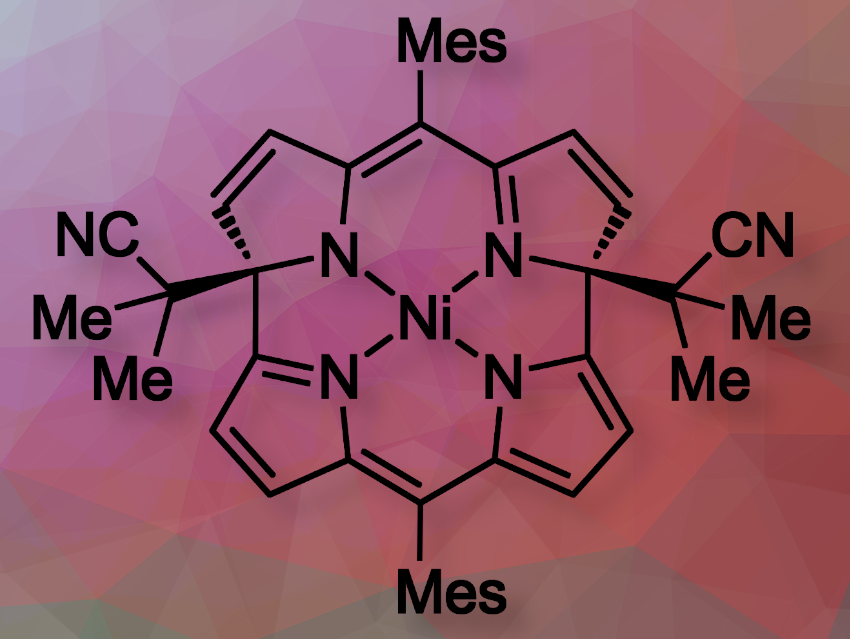
Hiroshi Shinokubo and colleagues, Nagoya University, Japan, have investigatted how Ni(II) norcorroles react with alkyl radicals from common azo initiators. The team used 2,2′-azobis(isobutyronitrile) (AIBN) as a radical source and treated Ni(II) dimesitylnorcorrole with AIBN in refluxing toluene. The reaction proceeded smoothly to afford a dialkylated macrocycle (pictured) in 92% yield. Additionally, a monoalkylated product and a dipyrrin dimer were obtained as minor products in 4% and 3% yields, respectively.
They researchers found that the reaction selectively occurs at the distal α-position, leading to nonaromatic bowl-shaped macrocycles. The antiaromatic character of the macrocycle changed to nonaromatic. This results in different light absorption and electrochemical properties compared to the original norcorrole.
The team also tested 1,1′-azobis(cyclohexane-1-carbonitrile) as a radical source, which yielded another dialkylated macrocycle with an 87% yield. However, alternative radical sources such as benzoyl peroxide, 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO), and alkyl halides with BEt₃ were ineffective.
DFT calculations showed that the SOMO of an isobutyronitrile radical (−5.98 eV), which was generated through denitrogenation of AIBN, is closer to the HOMO level of Ni(II) norcorrole (−4.68 eV) than to its LUMO (−3.16 eV). This explains the selective addition of the electrophilic isobutyronitrile radical to the distal α-position of the pyrrole unit. The spin density of the radical intermediate also governs regioselectivity.
This study uncovers previously unexplored radical reactions of Ni(II) norcorroles, broadening our understanding of their versatile chemistry. The researchers highlight that forming nonplanar macrocycles with unique properties demonstrates the potential to create novel functional materials from antiaromatic systems. These findings offer insights into regioselective radical additions, which could be used to design new porphyrinoid-based compounds with tailored characteristics.
- Radical reactivity of antiaromatic Ni(II) norcorroles with azo radical initiators,
Siham Asyiqin Shafie, Ryo Nozawa, Hideaki Takano, Hiroshi Shinokubo,
Beilstein J. Org. Chem. 2024, 20, 1967–1972.
https://doi.org/10.3762/bjoc.20.172