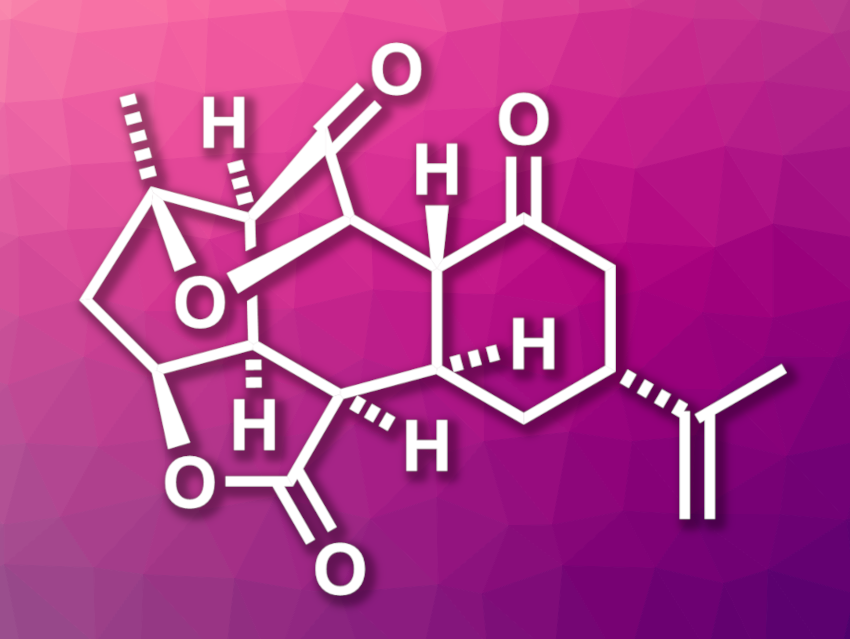
Ineleganolide (pictured) is a natural product that was isolated from a soft coral. It has a highly oxidized, stereochemically complex structure featuring a central seven-membered ring. This structure makes it an interesting and challenging target for total synthesis.
Brian M. Stoltz, California Institute of Technology, Pasadena, USA, and colleagues have developed a convergent and stereospecific synthesis of (+)-ineleganolide. The team’s approach is based on the preparation and coupling of two fragments, one derived from (−)-linalool that contains two five-membered rings and the other derived from (+)-norcarvone that contains the six-membered ring and the isopropenyl group. The seven-membered ring was constructed late in the synthesis.
To construct the linalool-based fragment, the team converted (R)-linalool to an aldehyde-functionalized cyclopentene derivative, followed by conversion of the aldehyde group to an epoxide and then to a iodohydrin. The second five-membered ring is then closed via an intramolecular substitution, followed by deprotection and oxidation steps.
The norcarvone-based fragment was obtained in three steps from (S)-norcarvone. The two fragments were coupled via esterification. A Michael addition and aldol cascade was then used to build a pentacyclic framework, followed by oxidation. Finally, a semipinacol rearrangement induced by samarium diiodide was used to introduce the central seven-membered ring. Overall, the team completed the total synthesis of (+)-ineleganolide in 23 steps (longest linear sequence) from (−)-linalool.
- A Convergent Total Synthesis of (+)-Ineleganolide,
Benjamin M. Gross, Seo-Jung Han, Scott C. Virgil, Brian M. Stoltz,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c02142