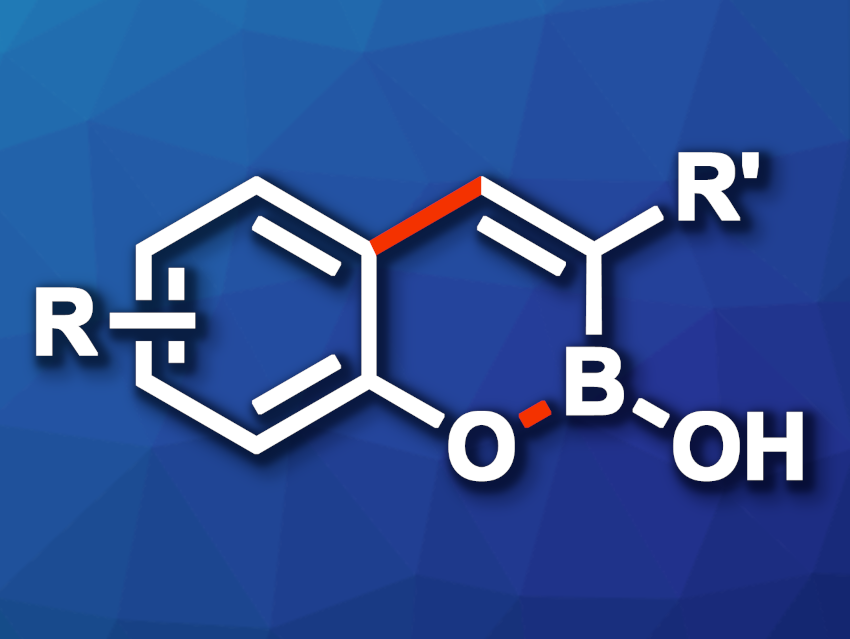
Bicyclic boronates can be interesting compounds, e.g., in medicinal chemistry. New methods for the synthesis of substituted analogues whose structure can be tuned to achieve suitable properties for drug development would be useful in this context.
Peter H. Seeberger, Max Planck Institute of Colloids and Interfaces, Potsdam, Germany, and Free University Berlin, Germany, John J. Molloy, Max Planck Institute of Colloids and Interfaces, and colleagues have developed a synthetic approach to 3-substituted bicyclic boronates (general product structure pictured) that is operationally simple, convergent, and has a good functional group tolerance. The team reacted 2-halophenol derivatives as one building block with vicinal boronic esters as the second building block. The transformation proceeds via a Suzuki–Miyaura cross-coupling/cyclization cascade.
The researchers used PdCl2dppf·DCM (dppf = 1,1′-bis(diphenylphosphino)ferrocene, DCM = dichloromethane) as a catalyst for the cross-coupling step, in which one of the neighboring boronic esters and the halogen substituent are used to couple the two building blocks. This is followed by a cyclization involving the phenol unit’s OH group and the second boronic ester under acidic conditions. This approach provided access to a broad scope of 3-substituted bicyclic boronates in mostly moderate to good yields, and it can be suitable for late-stage functionalizations.
- Convergent synthesis of bicyclic boronates via a cascade regioselective Suzuki–Miyaura/cyclisation protocol,
Alessandro Marotta, Hannah M. Kortman, Chiara Interdonato, Peter H. Seeberger, John J. Molloy,
Chem. Commun. 2024.
https://doi.org/10.1039/D4CC04653F