The direct activation of C–H bonds in organic molecules is useful for the synthesis of functionalized molecules in an atom-efficient manner. However, many C–H bonds are very stable, and their activation requires either harsh conditions or expensive transition metal catalysts. This type of C–H activation often occurs via oxidative addition to the catalytically active metal center. Achieving similar reactivity with abundant main group elements is difficult for most compounds. Constrained phosphorus(III) compounds can be an exception because they can switch between the +III and +V oxidation states.
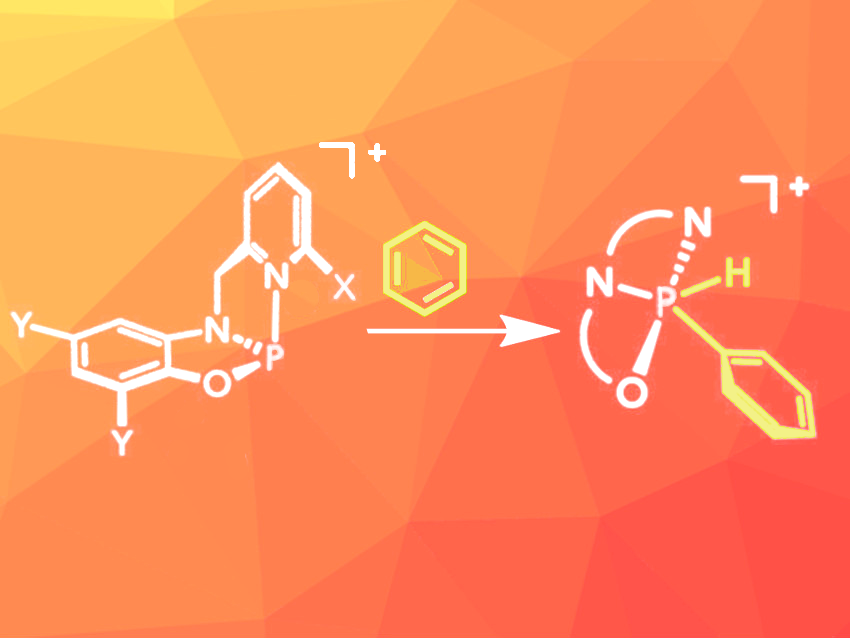
Lutz Greb, University of Heidelberg, Germany, Alexander T. Radosevich, Massachusetts Institute of Technology (MIT), Cambridge, USA, and colleagues have developed a series of constrained phosphenium ions based on easily accessible pyridinylmethylamidophenolate ligands that can activate C–H bonds in various alkynes and arenes, including nonactivated molecules such as benzene (pictured) and toluene. The ligands were synthesized from commercially available 2-pyridinecarboxaldehydes and 2-aminophenols. A reaction with phosphorus trichloride then provided phosphorus chloride intermediates in moderate to high yields. These intermediates were quantitatively converted to the desired phosphenium ions by dechlorination using alkali metal salts with weakly coordinating anions.
The oxidative addition of C–H bonds was initially tested with 1-methylindole and phenylacetylene, which both reacted at room temperature in deuterated dichloromethane. Initially, less reactive arenes such as thiophene were not converted, but after optimization of the substitution pattern at the ligand, this substrate was also converted at room temperature. For the addition of the even less reactive substrates benzene and toluene, the reaction mixture had to be heated to 100 °C and 70 °C, respectively. The oxidative addition proved to be reversible.
The team demonstrated that the reaction can be used for the functionalization of nonactivated arenes: Benzene was reacted with one of the developed phosphenium salts, and hydrolysis of the resulting benzene adduct provided access to phenylphosphinic acid.
- Reversible Oxidative Addition of Nonactivated C–H Bonds to Structurally Constrained Phosphenium Ions,
Daniel Roth, Alexander T. Radosevich, Lutz Greb,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c08456



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)