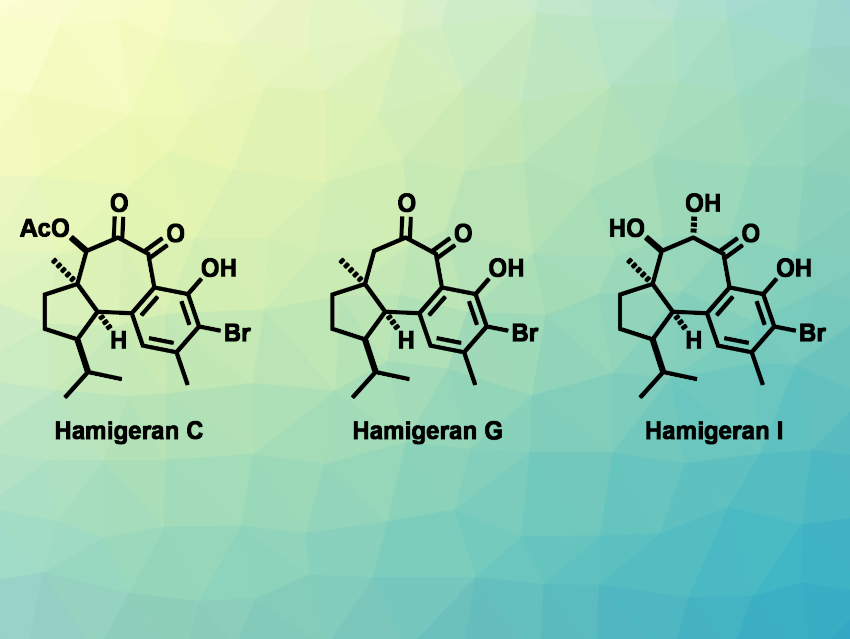
The hamigeran natural products were first isolated from the marine sponge Hamigera tarangaensis. They are a family of diterpenoids with interesting biological activities, e.g, antiviral or anticancer activities. Most of the 30 compounds in this family have a tricyclic 6–6–5 or 6–7–5 carbon skeleton. The latter group features a central seven-membered ring with multiple oxygenated carbons and contiguous stereocenters, fused to a polyfunctionalized aromatic ring and a cyclopentane ring. This complex structure can make the synthesis of these hamigerans challenging.
Mingji Dai, Purdue University, West Lafayette, IN, USA, and Emory University, Atlanta, Ga, USA, and colleagues have performed total syntheses of four hamigerans: hamigeran C, hamigeran G, debromohamigeran I, and hamigeran I. The team first prepared a tricyclic lactone, which was converted to a cyclopropanol derivative. This was followed by a palladium-catalyzed intramolecular cyclopropanol ring opening cross-coupling, which formed the central seven-membered ring.
The researchers then performed several oxidation reactions, including a challenging metal-free peroxide oxidation of aromatic C–H bonds, which was performed using Tomkinson’s reagent. An α-hydroxy ketone intermediate then served as the common starting point for the different desired products: A β-acetate elimination followed by hydrolysis and bromination gave hamigeran G, hydrolysis gave debromohamigeran I, which was brominated to give hamigeran I, and an oxidation, a selective hydrolysis, and bromination sequence gave hamigeran C.
Overall, the team achieved the total syntheses of hamigeran C in 14 steps, hamigeran G in 13 steps, debromohamigeran I in 12 steps, and hamigeran I in 13 steps. Apart from hamigeran G, the work represents the first total syntheses of these compounds.
- Concise Total Syntheses of the 6–7–5 Hamigeran Natural Products,
Baiyang Jiang, Mingji Dai,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c06031



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)