Ankit Saneja, Himalayan Bioresource Technology CSIR, Palampur, India, explains how he and colleagues have synthesized new conjugates of the chemotherapeutic agent gemcitabine and encapsulated a conjugate (4-decenoic acid-gemcitabine) in poly(lactic-co-glycolic acid) (PLGA) nanoparticles, significantly improving its anticancer efficacy in pancreatic and lung cancer cell lines.
What did you focus on in your recent ChemMedChem paper?
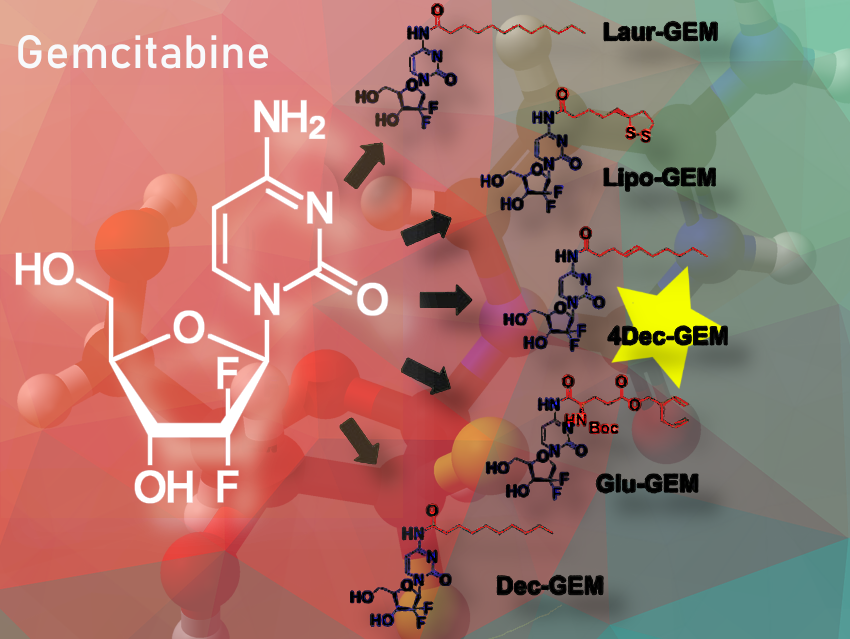
In this study, we have integrated the prodrug and polymeric nanoparticle approaches to improve the therapeutic efficacy of gemcitabine (GEM). Initially, we have synthesized five new conjugates of GEM by modifying the N-4 amino group of GEM with five different acids: 4-decenoic acid (4Dec), lipoic acid (Lipo), lauric acid (Laur), 5-benzyl N-(tert-butoxycarbonyl)-L-glutamate (Glu), and decanoic acid (Dec).
Among the synthesized conjugates, 4Dec-GEM demonstrated cytotoxic activity comparable to native GEM and improved plasma stability. To further enhance its therapeutic efficacy, 4Dec-GEM was encapsulated into poly(lactic-co-glycolic acid) (PLGA) nanoparticles using the single-emulsion method (as the synthesized conjugate was lipophilic in nature) using high-pressure homogenization. This combined prodrug and nanoparticle approach highlights the potential of integrating two approaches to improve the therapeutic efficacy of bioactive compounds.
Why is this necessary?
The rapid degradation of GEM presents significant challenges in maintaining effective drug concentrations in the bloodstream and tumor tissues over extended periods. GEM is quickly degraded in plasma, converting into its inactive metabolite (dFdU). This issue can be overcome by modifying the N-4 amino group of GEM, which mitigates GEM deamination through the prodrug approach.
These prodrugs are designed to undergo enzymatic and/or chemical transformation in the body, converting into active GEM, while minimizing premature degradation in the plasma. By shielding GEM from enzymatic degradation, these prodrugs may have the potential to improve GEM stability and enhance therapeutic outcomes.
Encapsulating 4Dec-GEM into poly(lactic-co-glycolic acid) (PLGA) nanoparticles offers the benefits of nanoparticle-based delivery systems, including improved drug protection, controlled release, and the potential for targeted delivery.
How did you detect the improved activity and minimized premature degradation in plasma?
The cytotoxicity of the developed PLGA nanoparticles was evaluated in A549, MIA-PaCa-2, and PANC-1 cancer cell lines, demonstrating improved cytotoxicity compared to native GEM and 4Dec-GEM. In vitro plasma stability of GEM and 4Dec-GEM was assessed by time-dependent degradation of GEM up to 24 hours in plasma using HPLC. The results revealed improved plasma stability of 4Dec-GEM compared to native GEM.
What is the longer-term vision for your research?
Our long-term vision is to create a versatile and effective platform to enhance the bioavailability and therapeutic efficacy of bioactive molecules by combining chemistry and formulation strategies. We believe that the synergy between chemistry, biology, and formulation approaches can lead to significant breakthroughs in treating various diseases. This study exemplifies an integrative approach, merging chemistry, biology, and formulation strategies to address challenges with therapeutic agents.
What part of your work was the most challenging?
There are various challenges in this study. Initially, we aimed to develop conjugates that were more potent than gemcitabine (GEM). The conjugates were synthesized in three main steps: First, the two hydroxyl groups of gemcitabine were protected with t-butyldimethylsilyl (TBDMS) groups by reacting GEM with imidazole and t-butyldimethylsilyl chloride (TBDMSCl) in N,N-dimethylformamide (DMF) at 0°C. The reaction mixture was stirred for 24 hours, followed by extraction with ethyl acetate (EtOAc), and purification via column chromatography, yielding the desired conjugate with an 86% yield.
In the second step, the TBDMS-protected gemcitabine was reacted with various acids in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), 1-hydroxybenzotriazole (HOBt), and N-methylmorpholine (NMM) in DMF at room temperature for 12 hours. Upon completion, the reaction mixture was extracted with EtOAc and purified by column chromatography. Finally, the TBDMS groups were deprotected by using tetrabutylammonium fluoride (TBAF). The crude product was then purified by column chromatography to obtain the final gemcitabine conjugates.
The most challenging steps were the initial protection of the two hydroxyl groups of GEM with t-butyldimethylsilyl (TBDMS) groups, followed by deprotection using tetrabutylammonium fluoride (TBAF), which limited the yield of the synthesized conjugates.
Additionally, encapsulating 4Dec-GEM into PLGA nanoparticles using the single-emulsion method required rigorous optimization of formulation parameters and high-pressure homogenization to achieve the desired particle size.
Thank you very much for sharing these insights.
The paper they talked about:
- Synthesis of a Gemcitabine Prodrug and its Encapsulation into Polymeric Nanoparticles for Improved Therapeutic Efficacy,
Kajal Kaliya, Neha Bhardwaj, Ruchika, Ankit Saneja,
ChemMedChem 2025.
https://doi.org/10.1002/cmdc.202400532

Ankit Saneja is a Senior Scientist at the CSIR – Institute of Himalayan Bioresource Technology in Palampur, Himachal Pradesh, India.