Resistances against commonly used antimalarial drugs have resulted in a surge in the number of malaria cases in recent years. This emerging resistance has posed a significant challenge to malaria control programs. Thus, the discovery of new drug candidates with new mechanisms of action is important.
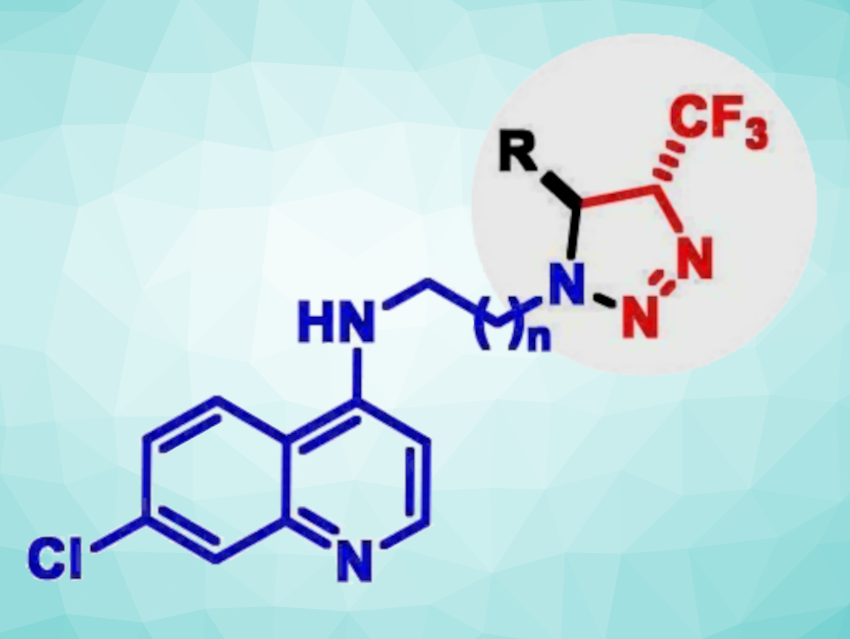
Kishor Mohanan, Renu Tripathi, CSIR-Central Drug Research Institute (CSIR-CDRI), Lucknow, India, and colleagues have identified a new class of 4-aminoquinoline-trifluoromethyltriazoline compounds (general structure pictured) as possible antiplasmodial agents (i.e., compounds that are active against the parasite that causes malaria). A small library of these compounds was prepared via a silver-catalyzed three-component reaction of trifluorodiazoethane with a Schiff base generated in situ from the corresponding quinolinyl amines and aldehydes.
A series of 32 compounds was tested against both chloroquine-sensitive and chloroquine-resistant strains of the malaria parasite. Chloroquine phosphate is used to prevent and treat malaria. Among them, four compounds were found to be potent with IC50 values of 4–20 nM against a chloroquine-sensitive and 120-450 nM against a chloroquine-resistant strain, respectively. One of these candidates was also found to be effective in a mouse model, showing a 99.9 % decrease in parasitic load at day 7 post-infection, along with a 40 % cure rate. Overall, the work indicates that the incorporation of a trifluoromethylated triazoline unit can lead to promising antiplasmodial activity in this compound class.
- Incorporation of Trifluoromethyltriazoline in the Side Chain of 4‐Aminoquinolines: Synthesis and Evaluation as Antiplasmodial Agents,
Kanchan Yadav, Anamika Sharma, Salique Hassan Shaham, Sanoop P Chandrasekharan, Sandeep Kumar, Anamika Dhami, Hisana Nasreen, Kishor Mohanan, Renu Tripathi,
ChemMedChem 2023.
https://doi.org/10.1002/cmdc.202200653



Congratulations, it’s great work