Uranium, especially in the U(VI) redox state, is important in the nuclear industry. U(VI)-containing wastewater is an environmental hazard. In addition, U(IV) can be scarce, so capturing it from wastewater or seawater would be useful. Adsorption is a promising approach due to its low cost and simplicity, but requires efficient and selective adsorption materials for the low U(VI) concentrations.
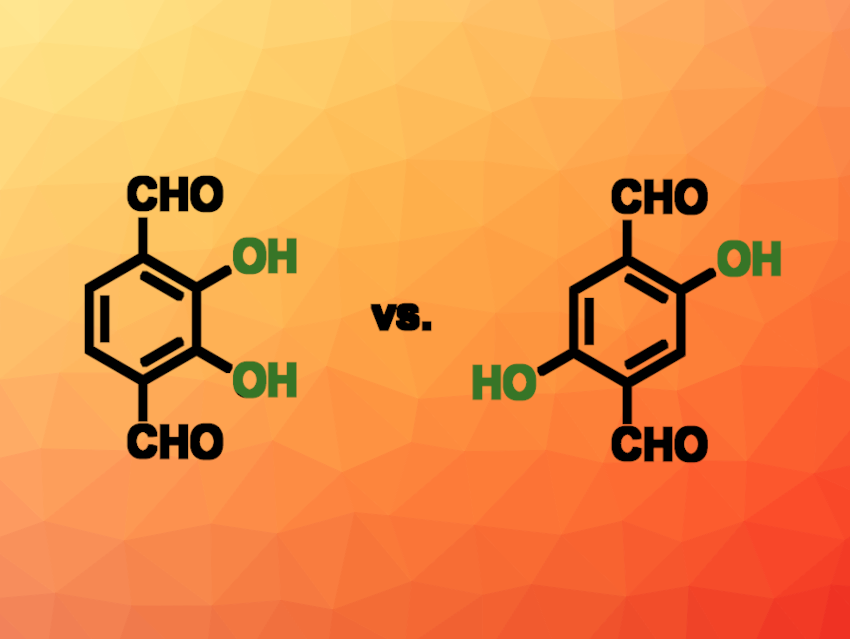
Yong Zhang, Southwest University of Science and Technology, Mianyang, China, Yongwu Peng, Zhejiang University of Technology, Hangzhou, China, and colleagues have developed two covalent organic frameworks (COFs), named TAPA-COF-1 and TAPA-COF-2, that form nanosheets for use in uranium capture. The two COFs differ in the arrangement of hydroxyl groups: They are synthesized using 2,3-dihydroxyterephthalaldehyde (2,3-Dha, pictured above on the left) for TAPA-COF-1 and 2,5-dihydroxyterephthalaldehyde (2,5-Dha, pictured above on the right) for TAPA-COF-2. These aldehydes were reacted with tri(4-aminophenyl)amine (TAPA) to form the desired COFs.
The team found TAPA-COF-1 nanosheets perform better than TAPA-COF-2 nanosheets in the capture of U(VI). They attribute this to the arrangement of hydroxyl groups in the frameworks: Due to the neighboring OH groups, TAPA-COF-1 features more chelating sites. This optimized COF has an U(VI) uptake capacity of 657.2 mg g–1. According to the researchers, TAPA-COF-1 could be a promising adsorbent for U(VI) capture.
- Tailoring Chelating Sites in Two-Dimensional Covalent Organic Framework Nanosheets for Enhanced Uranium Capture,
Ying Huang, Jun Liao, Jiahao Li, Changming Cheng, Yong Zhang, Yongwu Peng,
Chem. Commun. 2024.
https://doi.org/10.1039/D3CC05125K



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)