The selective addition of Si–H motifs to a C≡C bond can be challenging as it is necessary to control the process in terms of regio-, stereo-, and chemoselectivity. This type of reaction can, e.g., be used to prepare vinylsilanes, which can serve as intermediates in various synthetic transformations. Often, their synthesis relies on the use of noble-metal catalysts. The development of alternative catalysts based on Earth-abundant metals would, thus, be useful.
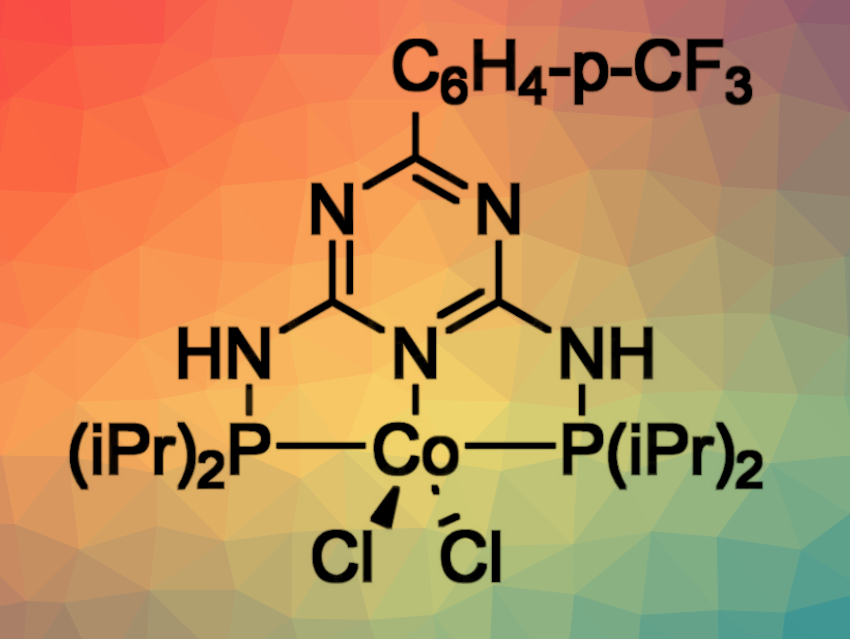
Grzegorz Hreczycho, Adam Mickiewicz University, Poznań, Poland, Rhett Kempe, University of Bayreuth, Germany, and colleagues have developed a selective protocol for the syn-hydrosilylation of internal alkynes (pictured below). The reaction is catalyzed using inexpensive and readily available cobalt complexes with triazine-based PNP-type pincer ligands (pictured above). The reactions were performed in tetrahydrofuran (THF) .
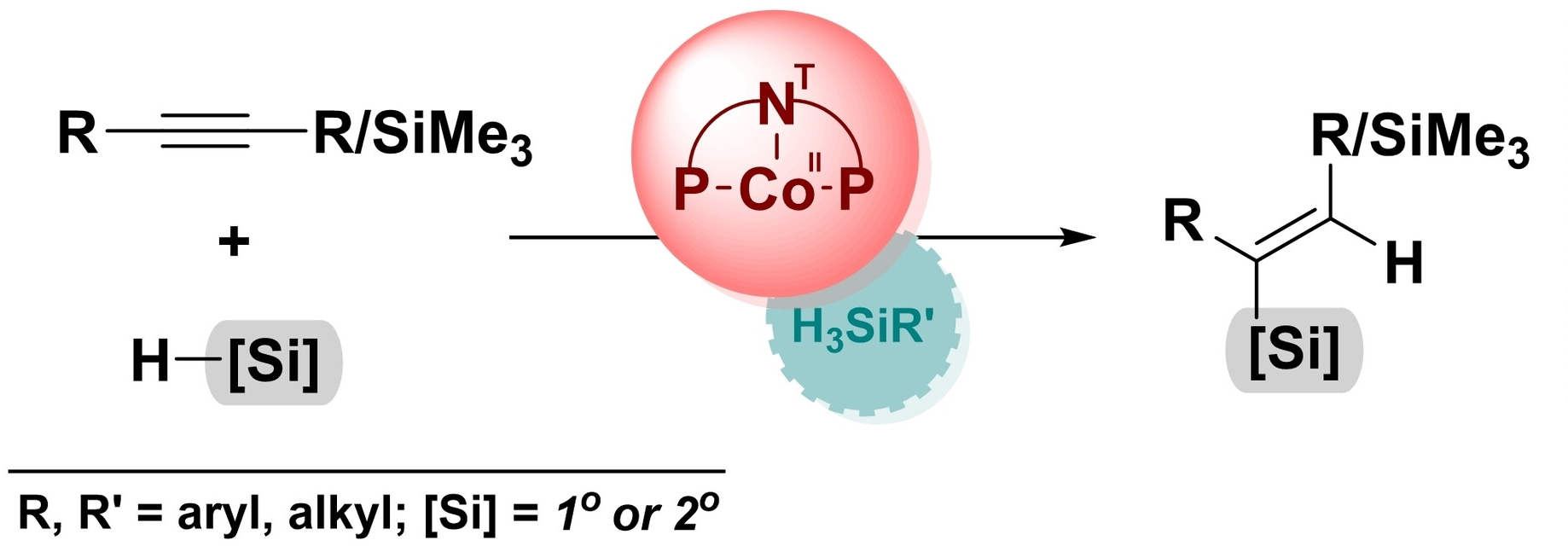
The developed method provides an efficient and selective approach to obtain (E)-silylalkenes and vicinal disilylalkenes under mild reaction conditions. It has a broad substrate scope, including both aliphatic and aromatic acetylenes with electron-donating or electron-withdrawing substituents on the phenyl ring, as well as primary and secondary silanes. Unlike some other transition-metal-catalyzed procedures, no external additives were required since the hydrosilanes act as both substrates and activators. This reduces the environmental impact and process costs.
- Co‐catalyzed Selective syn‐Hydrosilylation of Internal Alkynes,
Hanna Stachowiak-Dłużyńska, Krzysztof Kuciński, Bożena Wyrzykiewicz, Rhett Kempe, Grzegorz Hreczycho,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202300592