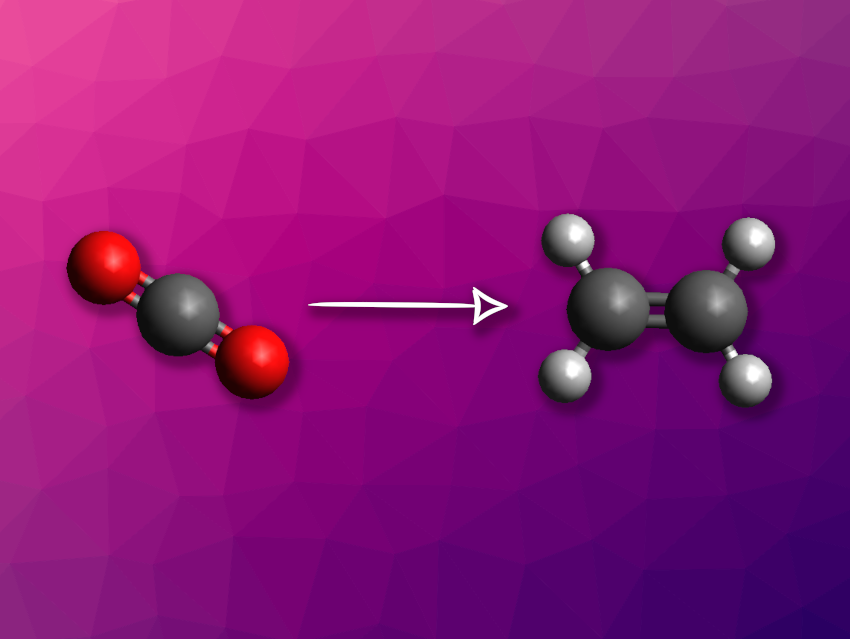
Using the greenhouse gas CO2 as a chemical feedstock can be useful to reduce the dependence on fossil resources and curb emissions. Reducing CO2 to valuable chemicals such as ethylene (ethene) electrochemically using renewable energy is one option for this. The selective formation of ethylene, which involves a carbon–carbon coupling, requires optimized conditions with a suitable electrocatalyst.
Ruixiang Li, Sichuan University, Chengdu, China, Jiaqi Xu, Sichuan University and École Polytechnique Fédérale de Lausanne (EPFL), Switzerland, and colleagues have used crown ethers to functionalize copper-based metal–organic frameworks (MOFs) with the aim of designing electrocatalysts for the generation of ethylene from CO2. The crown ether interacts with specific cations, changing their concentration at the catalyst surface and promoting the desired reaction. Specifically, the team used 18-crown-6 to achieve K+ coordination, with the potassium ions then promoting the desired dimerization of C1 intermediates on the catalyst surface.
The researchers tried different copper-based MOFs, such as CuBTC (copper(II) benzene-1,3,5-tricarboxylate), which were dissolved in isopropanol together with 18-crown-6 and the polymer Nafion-117 and then applied onto an electrode substrate to create a working electrode. They found that the modification with the crown ether improved the activity for CO2 reduction and shifted the preferred product from CO to ethylene. The team attributes this to the formation of a catalytically active Cu2O phase, which is stabilized by the crown ether, as well as to the coordination of K+ ions on the catalyst surface promoting the adsorption of a *CO intermediate and the C–C coupling.
- Crown ether functionalization boosts CO2 electroreduction to ethylene on copper-based MOFs,
Xuan Zheng, Siheng Yang, Dingwen Chen, Yuxuan Kong, Tianhua Cui, Xueli Zheng, Haiyan Fu, Weichao Xue, Shuang Li, Chong Cheng, Hua Chen, Ruixiang Li, Jiaqi Xu,
Chem. Commun. 2025.
https://doi.org/10.1039/D4CC06719C