Plastic waste is an environmental problem, and the majority of plastics are produced from limited fossil resources. Creating chemically recyclable polymers can be useful to tackle both of these problems. Diols, for example, are readily available and sustainable building blocks. They are used as raw materials for the synthesis of polyesters. The development of new, diol-derived, sustainable polymers is an interesting research target.
Xinghong Zhang, Zhejiang University, Hangzhou, China, and colleagues have developed a new click reaction that can be used to connect alcohols with activated alkenes via a carbonyl sulfide (COS) bridge. COS is a waste product, and its use for manufacturing could, thus, be useful. The team used it in a step polyaddition of diols, diacrylates, and COS to synthesize a series of chemically recyclable poly(thiocarbonate esters) (pictured). The ester and thiocarbonate groups in the polymer chain can serve as breakpoints and enable the polymers to be chemically recycled.
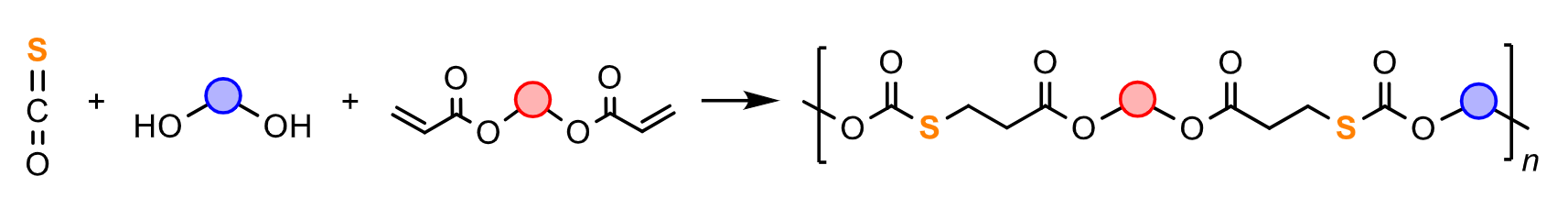
The team used commercially available diols such as poly(ethylene glycols), neopentyl glycol, and α,w-aliphatic diols and reacted them with their corresponding diacrylates and COS in the presence of 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU) as a catalyst at 60 °C. The desired polymers were obtained in high yields. The developed method is efficient and atom-economical, and it proceeds under mild conditions. The polymers can be chemically recycled via hydrolysis, esterification, and another step polymerization.
- Modular alcohol click chemistry enables facile synthesis of recyclable polymers with tunable structure,
Yanni Xia, Yue Sun, Ziheng Liu, Chengjian Zhang, Xinghong Zhang,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202306731
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)


