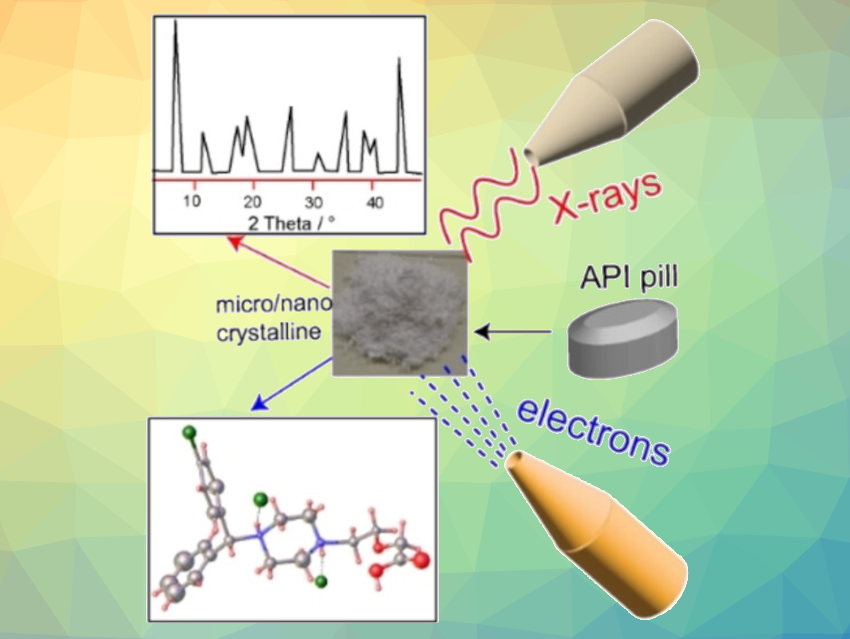
For pharmaceuticals, knowing the chemical composition is not enough—molecular geometry and crystal structure also play important roles in a drug’s activity. With a method based on electron diffraction, Durga Prasad Karothu, New York University Abu Dhabi, United Arab Emirates, Panče Naumov, New York University Abu Dhabi and New York University, USA, have determined the structure of the antihistamine Levocetirizine dihydrochloride. The advantage of this technique is that, unlike for X-ray crystallography, nanoscale crystals are sufficient.
Structures of Active Pharmaceutical Ingredients
Despite being chemically identical, many pharmaceutical substances can adopt different crystal structures or form cocrystals with an additive. This can significantly influence the properties of a drug, such as bioavailability, solubility, stability, and tabletability. Structural determinations are, thus, important in the development of advanced solid pharmaceuticals.
Today, the standard, routine method for determining the three-dimensional structures of crystalline molecules and biological macromolecules with atomic resolution is single-crystal X-ray diffraction structure analysis (SCXRD). The atoms within the crystal diffract the X-ray radiation, forming a diffraction pattern from which the positions of the individual atoms in the structure of the crystal can be calculated. This requires sufficiently large, well-diffracting single crystals. However, many compounds are difficult or impossible to crystallize.
An alternative method is powder X-ray diffraction (PXRD), which can analyze a sample in the form of a powder. However, the data analysis is not straightforward and if the sample is a mixture of several phases of the same or different compounds, it is very difficult and often ambiguous.
Electron Diffraction as an Alternative
A more recent technique is 3D-electron diffraction/micro-crystal diffraction (3D ED/MicroED). Instead of X-rays, electron beams from an electron microscope are diffracted. Because the interaction of matter with electrons is significantly stronger than interactions with X-rays, sub-micro to nanometer-sized crystals produce diffraction patterns that can be evaluated, and the direct analysis of components in microcrystalline mixtures becomes possible.
The team used 3D ED/MicroED to determine the structure of Levocetirizine dihydrochloride. Levocetirizine is an over-the-counter oral antihistamine used to treat allergy symptoms such as hay fever and hives. Although it has been in broad use, its crystal structure has remained unknown because no crystals that are “good enough” for X-ray crystallographic analysis could be grown. Recently, the structure of this medication was studied using powder X-ray diffraction and computer calculations—but uncertainty and ambiguity remained.
The researchers worked with crystals obtained by grinding commercially available tablets. They collected a total of 20 datasets based on different crystallites. The datasets identified as Levocetirizine dihydrochloride revealed a non-centrosymmetric monoclinic space group P21 with two molecules in the asymmetric unit.
In addition to determining the drug’s crystal structure, they were able to use dynamical refinement of the structure to unambiguously determine the absolute configuration of the Levocetirizine. The R-form was assigned.
Powerful Strategy For Resolving Micro- or Nanocrystallites
The team also found diffraction patterns for other constituents of the tablets, such as lactose monohydrate. Due to the collection of data on a single-grain level, 3D ED/MicroED can be used to find the structure of the active pharmaceutical ingredient even in the presence of large amounts of other constituents.
Overall, the work demonstrates the potential of 3D ED/MicroED for the structure elucidation of components of microcrystalline mixtures without a need to grow large single crystals.
- The Elusive Structure of Levocetirizine Dihydrochloride Determined by Electron Diffraction,
Durga Prasad Karothu, Zainab Alhaddad, Christian R. Göb, Christian J. Schürmann, Robert Bücker, Panče Naumov,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202303761