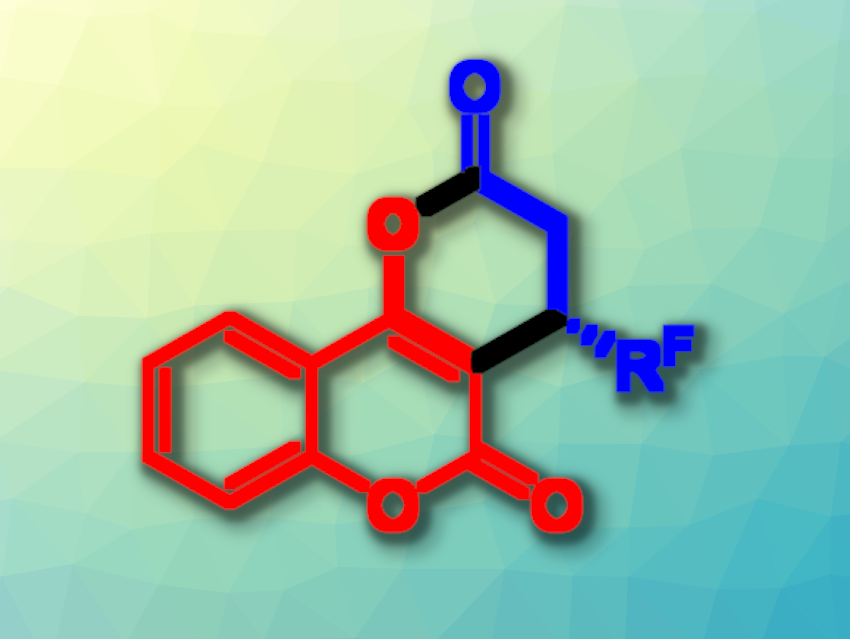
Chiral oxygen-containing heterocycles are often found as structural motifs in natural products, pharmaceuticals, and bioactive compounds. Oxygen-heterocycle-fused coumarins, for example, are featured in a variety of natural products. Methods for their asymmetric preparation can be useful, e.g., in organic synthesis or drug development.
Feng Shi, Jiangsu Normal University, Xuzhou, China, and colleagues have developed a method for the enantioselective isothiourea-catalyzed asymmetric (3+3) cycloaddition of 4-hydroxycoumarins with β-fluoroalkyl-substituted α,β-unsaturated aryl esters, giving chiral fluoroalkyl-substituted caprolactone-fused coumarins (general product structure pictured). The team used a chiral isothiourea as an organocatalyst, PhCOOH as an acid additive, and CHCl3 as the solvent. The reactions were performed at 25 °C.
Using this approach, the desired products were obtained in moderate to good yields (up to 88 %) and mostly excellent enantioselectivities (up to 98 % ee). The team proposes a reaction mechanism that involves the reversible conversion of the β-fluoroalkyl-substituted α,β-unsaturated aryl ester into an α,β-unsaturated acyl ammonium intermediate by the catalyst, an enantioselective addition to the 4-hydroxycoumarin, and an intramolecular cycloaddition.
- Organocatalytic Asymmetric (3+3) Cycloaddition of 4‐Hydroxycoumarins with α,β‐Unsaturated Aryl Esters,
Hao Chen, Shuang Yang, Ning-Yi Wang, Ya-Ping Tian, Yu-Chen Zhang, Feng Shi,
ChemCatChem 2025.
https://doi.org/10.1002/cctc.202401925