Despite significant progress, achieving chiral systems with exceptional chiroptical performance remains a major challenge. Understanding how chirality spreads and dissymmetry amplifies at the molecular level is crucial for developing such systems.
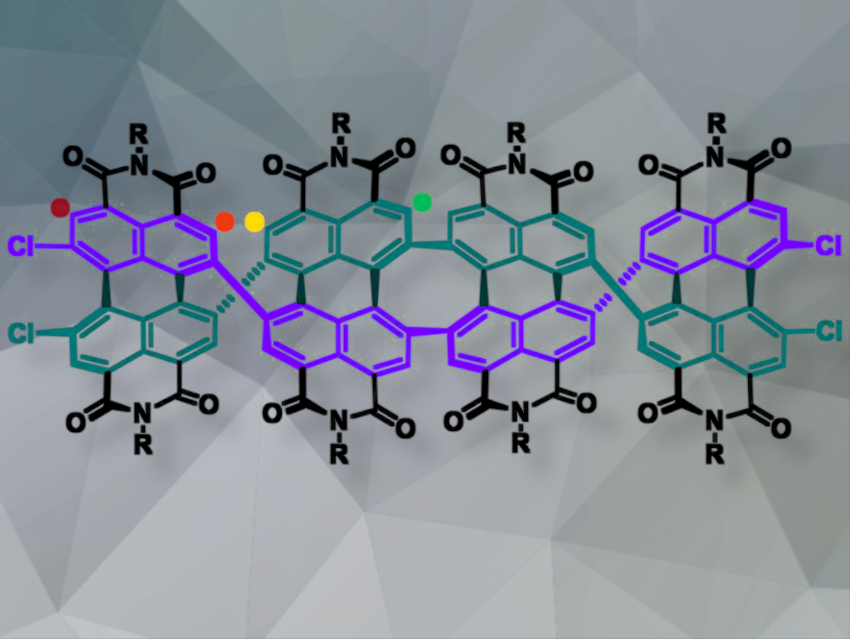
Wei Jiang and colleagues, Tsinghua University, Beijing, China have developed a simple Cu-mediated Ullmann homocoupling method to synthesize double π-helical nanoribbons based on perylene diimide (PDI). These nanoribbons form dimers, trimers, and tetramers (example pictured) with either homochiral or heterochiral linking of chiral centers. The team reacted N,N’-di(2,6-diisopropylphenyl)-1,6,7,12-tetrachloroperylene-3,4:9,10-tetracarboxylic diimide (4ClPDI) with Cu and Pd(PPh3)4 in dry DMSO under argon. They found that a controlled strategy, including regulating reaction concentration, results in the formation of dimers and trimers, with the possibility of further coupling to generate higher oligomers.
The molecular design incorporates linearly extended double π-helices with cyclooctatetraene (COT) as the chiral centers and PDI as the conjugated units. The molecules provide a platform to understand how chirality spreads and dissymmetry amplifies.
The team found that extending the helix increases the chiroptical responses in the ground and excited states. Furthermore, the arrangement of the chiral centers significantly influences chiral propagation. Homochiral tetramers showed higher magnetic transition dipole moment densities than heterochiral tetramers, favoring higher absorption dissymmetry factors.
These double π helices showed exceptional photoluminescence quantum yields (ΦPL) ranging from 83 to 95%. The circularly polarized luminescence brightness (BCPL) of the homochiral tetramer reached 575 M–1 cm–1, representing one of the highest values reported for chiral small molecules.
- Highly Luminescent Chiral Double π-Helical Nanoribbons,
Yujian Liu, Zuoyu Li, Ming-Wei Wang, Jiangtao Chan, Guogang Liu, Zhaohui Wang, Wei Jiang,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c11942