Aniello Palma, University College Dublin, Ireland, talks about a paper recently published in ChemistryEurope that describes the design principles of metallo-organic ligands applied to polyproline peptides, leading to the synthesis of a stable, stereoselective Pd lantern cage in water.
What did you do?
Metallo-organic cages are a class of discrete supramolecular non-covalent constructs for which a large set of assembly rules has been successfully established. These discrete structures find applications in host-guest chemistry, catalysis, and drug delivery, to mention some.
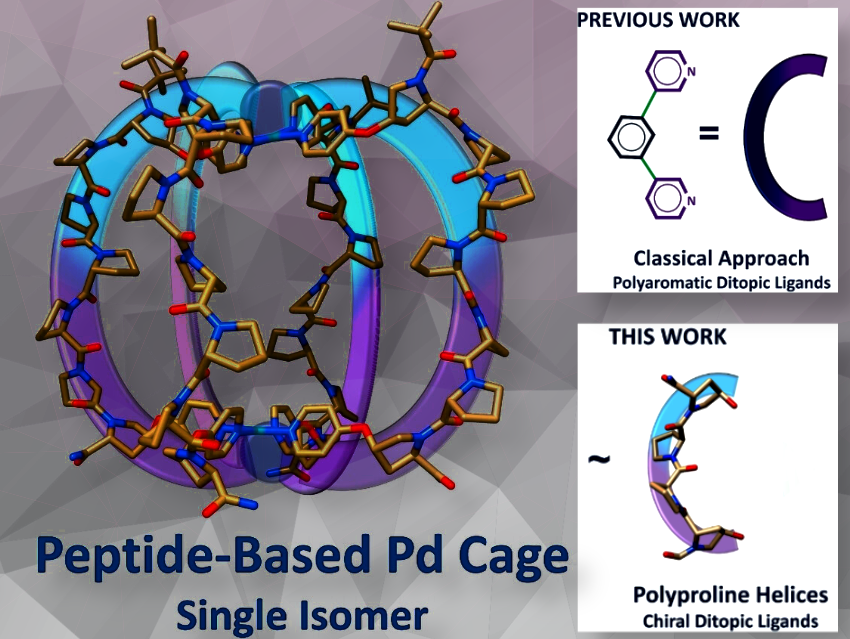
We successfully demonstrated that the same assembly rules can be applied to a particular class of peptides with a defined secondary structure: the polyproline helix.
Remarkably, when treated with a palladium salt, we obtained only a single species in solution out of four potential metallo-cage isomers. The resulting peptide-based Pd cage showed excellent stability in water and the ability to form a host-guest complex with a toxic and reactive anion generated in situ.
Why are you interested in this?
We are particularly interested in developing novel bio-compatible functional supramolecular constructs using peptides as building blocks. While α-helices and β-sheets have been successfully exploited as supramolecular building blocks, the polyproline helix, a different type of secondary structure found in peptides, has been largely overlooked.
We have recently demonstrated the resilience of this secondary structure and shown that it is possible to predict, with high accuracy, the spatial position of chemical handles introduced onto the polyproline backbone.
Our team aims to establish the use of polyproline helices as a powerful supramolecular building block and this work is an important stepping stone towards this goal.
What is new and cool about your work?
What is cool about this work is that we were able to predict the behavior of the functionalized polyproline. This is not trivial, as small peptides are typically not structured.
Based on our previous work, which includes a several solid-state structures of different polyprolines, we were confident that the spatial arrangement of the chemical handles on the polyproline would mimic the classical “C” shape of ditopic ligands used in the synthesis of lantern-shaped Pd²⁺ cages.
The fact that the chiral helical polyproline yielded a single isomer in solution is also remarkable.
The ability of this novel chiral and biocompatible peptide-based cage to perform host-guest chemistry in water is also an important achievement, and we are confident that these results will open new and exciting research avenues.
What is the main significance of your results?
We were excited to see the chiral peptide-based ligand yielding a single isomer in solution as this result meant that these types of cages can be used as a starting point towards the future design of more complex functionalized materials.
The cage stability in water over months at room temperature is also a significant finding as this demonstrates the stability of these types of constructs in the view of future applications.
What specific applications do you imagine?
As the ability to perform host-guest chemistry has been demonstrated for this class of peptide-based cages, we anticipate a move towards the synthesis of more complex and functional cages with potential applications in asymmetric catalysis, drug delivery, and chemical separation.
What part of your work was the most challenging?
The real challenge associated with this work was the identification of the right class of structured peptide to use. We needed to identify a peptide secondary structure resilient to the introduction of chemical handles.
In fact, the use of other structured peptides, such as α-helix and β-sheet peptides, would have been challenging, as they can suffer perturbation of their periodicity upon functionalization (i.e. deterioration of their secondary structures). Our previous work on polyproline helices as supramolecular building blocks inspired the idea exploited in this work.
Thank you very much for sharing these insights.
The paper they talked about:
- Applying Metallo-Organic Ligand Design Principles to the Stereoselective Synthesis of a Peptide-Based Pd2L4X4 Cage,
Dominic F. Brightwell, Kushal Samanta, Jimmy Muldoon, Patricia C. Fleming, Yannick Ortin, Lina Mardiana, Paul G. Waddell, Michael J. Hall, Ewan R. Clark, Felipe Fantuzzi, Aniello Palma,
ChemistryEurope 2024.
https://doi.org/10.1002/ceur.202400050
Aniello Palma is an Assistant Professor in Organic and Medicinal Chemistry at the School of Chemistry, University College Dublin, Ireland. His lab’s research is at the interface of chemistry, biology and materials science, focusing on the design of smart and responsive materials made using supramolecular approaches and inspired by biological polymers.