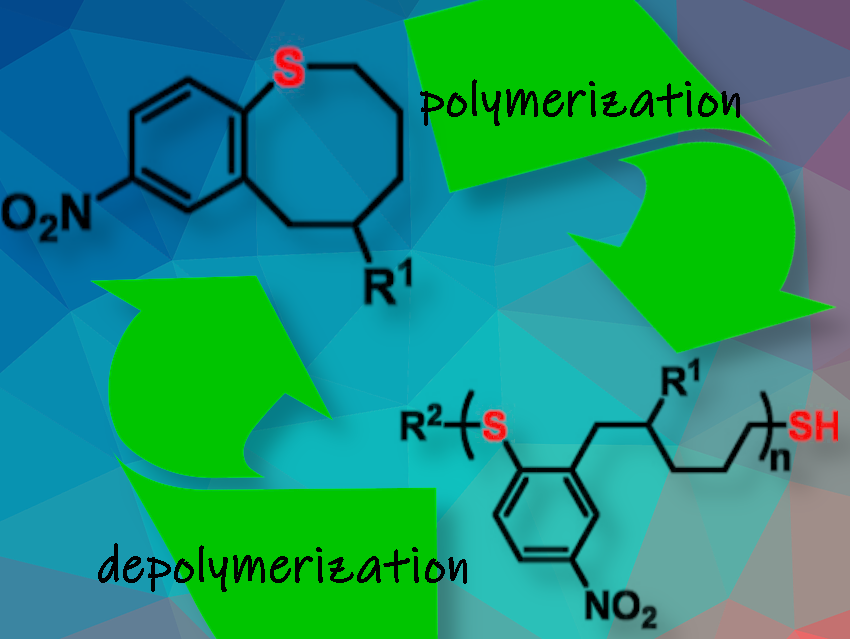
The essence of chemical recycling lies in the ability to break down a polymer into its original building blocks, or monomers. However, such chemical recycling is only economically and environmentally feasible, if it can be carried out under mild conditions and results in high yields of monomers. Most polymers that have been around for years cannot be easily degraded, much less depolymerized for complete monomer recovery.
A research group led by Will R. Gutekunst at the Georgia Institute of Technology in Atlanta, GA, USA, has introduced a recyclable polymer system based on the ability of sulfides to perform nucleophilic aromatic substitutions [1]. Using cyclic aromatic sulfides in ring-opening polymerization (ROP), the team succeeded in producing a high-performance polythioether thermoplastic with tunable functionality and chemical recyclability.
Reversing ROP – It’s the Temperature
Many researchers believe that ROP is the most promising route to fully reversible polymer systems. This belief stems from a thermodynamic perspective on polymerization: the enthalpy change driving the reaction is smaller when cyclic monomers open up for chain propagation. In this case, heating the system above a certain (“ceiling”) temperature would make the entropy term dominant, favoring monomers over polymers, and reverse the polymerization.
A similar mechanism also drives dynamic covalent networks in which covalent bonds reorganize as a response to triggers such as temperature or mechanical activation. As aryl sulfides can form such dynamic polymer networks based on nucleophilic aromatic substitution, Gutekunst and his colleagues explored cyclic aryl sulfides undergoing nucleophilic aromatic substitution as a starting point for reversible ROP.
Aryl Sulfide Based Thermoplastics
The team designed a cyclic aromatic thioether, benzothiocane, in which the aromatic carbon carrying the thioether group is electron-depleted. Under basic conditions, any incoming thiol, such as the initiator molecule dodecanethiol, would attack this electron-depleted site and eventually replace the sulfur atom of the cyclic aromatic thioether. The ring opens, and chain propagation by nucleophilic aromatic substitution begins. To terminate polymerization, the system simply needs to be acidified.
The researchers explain that the benzothiocane ring-opening polymerization works so well because the eight-membered thioether ring is strained enough to be easily broken open (having a larger enthalpy change), while the aromatic ring carries an electron-withdrawing group to provide electron depletion in the sulfidic carbon. Despite the effort required to design this polymerizable monomer by balancing reactivity and thermodynamics, the researchers assert that the actual benzothiocane molecule was fairly easy to make using well-known reactions and accessible starting materials.
Polymerization conditions included a base, a polar solvent, a thiol initiator, and a catalyst, resulting in a polythioether product with targetable molecular weight and variable functional groups. The material showed thermoplastic behavior and tensile performance “within the range of some commonly used thermoplastics, such as polyethylene, ethylene–vinyl acetate, and poly(vinyl acetate),” according to the paper. This suggests a variety of potential everyday applications, especially since recyclability is increasingly being sought by consumers and producers alike.
Chemical Recycling
Chemical recycling was made possible by using the reverse ROP strategy, i.e., adding more thiols to break up the backbone, increasing the temperature, and diluting. 85 % of the monomers were recovered, say the researchers. As the cyclic monomer is easy to make and can be equipped with extra functionalities, the benzothiocane approach could be further developed into a versatile platform for novel chemically recyclable, sulfide-based plastics.
To date, no chemically recyclable polymer has made the leap from laboratory to industrial scale. However, novel approaches to ROP could make a difference to the recyclable plastics landscape in the future.
Reference
[1] Y.-L. Su, L. Yue, H. Tran, M. Xu, A. Engler, R. Ramparasad, H.J. Qi, W. R. Gutekunst, Chemically Recyclable Polymer System Based on Nucleophilic Aromatic Ring-Opening Polymerization, J. Am. Chem. Soc. 2023. https://doi.org/10.1021/jacs.3c03455