Charles Friedel was born on March 12, 1832, in Strasbourg, France [1–4]. He is well-known for co-discovering the Friedel–Crafts reactions with James Mason Crafts, which are often used in organic synthesis to introduce alkyl or acyl groups at aromatic substrates with the help of a Lewis acid catalyst. Friedel worked as both a mineralogist and a chemist.
Friedel’s Career and Honors
Charles Friedel studied natural sciences in Strasbourg and then mathematics and physics at the Sorbonne in Paris, France. From 1956 to 1870, he worked as curator of the mineral collection at the École des Mines, Paris (today Mines ParisTech). Starting in 1854, he also studied chemistry under Charles Adolphe Wurtz at the École de Médecine, Paris. Wurtz was another chemist from the Alsace region who is known today for the Wurtz reaction, a coupling reaction between two alkyl halides using sodium metal.
Friedel was involved in the founding of the Société Chimique de France (SCF) in 1857, and he later served four terms as President of the society. He obtained his doctorate in 1869, with two completed theses: one on the chemistry of ketones and aldehydes and one on the pyroelectric properties of crystals—demonstrating how his focus was divided between chemistry and mineralogy.
Friedel started to work as a Lecturer in Mineralogy at the École Normale Supérieure, Paris, in 1871. He was appointed Professor of Mineralogy at the Sorbonne in 1876, and in 1884, he became the successor of Wurtz as Professor of Organic Chemistry at the Sorbonne. Charles Friedel passed away on April 10, 1899, in Montauban, France.
Among other honors, Friedel became Chevalier of the Legion of Honor (a French order of merit) in 1869 and was promoted to Officier in 1888. In 1880, he received the Davy Medal of the Royal Society, UK. He was a Member of the French Académie des Sciences, a Corresponding Member of the Bavarian Academy of Sciences, Germany, and the Russian Academy of Sciences in Saint Petersburg, a Foreign Member of the English Chemical Society, an Associated Member of the Académie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique, Belgium, and an Honorary Member of the German Chemical Society and the Royal Society of Sweden.
Mineralogy, Carbonyl Compounds, and Organosilicon Compounds
Friedel started out working in mineralogy and crystallography, and in his position as curator at the École des Mines, he strived to grow this important mineral collection. He was also interested in optics and astronomy, but ultimately was pulled to the chemical side of mineralogy as well as chemistry itself [3].
In Wurtz’s laboratory, Friedel worked on the chemistry of ketones and aldehydes, as well as the first investigation of the properties of secondary alcohols, in addition to other topics in organic chemistry. In 1861, Friedel first met the American chemist James Mason Crafts in Wurtz’s laboratory. The two first worked together on silicon chemistry, starting in 1863 [4]. They originally aimed to determine the atomic weight of Si, which was a topic of debate at the time, with some researchers claiming the formula of silica was SiO3, while others used SiO2. The tetravalent nature of the element was confirmed by the synthesis of a range of silicon compounds, including SiCl4, Si(OEt)4, and SiEt4 as the first organosilicon compound [5].
The Friedel–Crafts Reactions
Friedel and Crafts developed the Friedel–Crafts reactions in 1877 [6,7]. The two researcher’s discovery was accidental: They aimed to study the effects of metallic aluminium on amyl chloride (monochlorinated derivatives of the isomers of pentane) and observed an “abundant” evolution of HCl and an unexpected formation of new hydrocarbons [8]. They attributed this to the formation of aluminium chloride and then tested whether aluminium chloride can be used directly to induce the formation of novel hydrocarbons—which it could.
Based on this discovery, the two researchers used aluminium chloride for the development of a general method for the coupling of an organic halogenide with a hydrocarbon, in particular, benzene derivatives. This type of reaction is still widely used today to attach carbon substituents to aromatic substrates, either alkyl groups in the Friedel-Crafts alkylation or acyl units in the Friedel-Crafts acylation (pictured below).
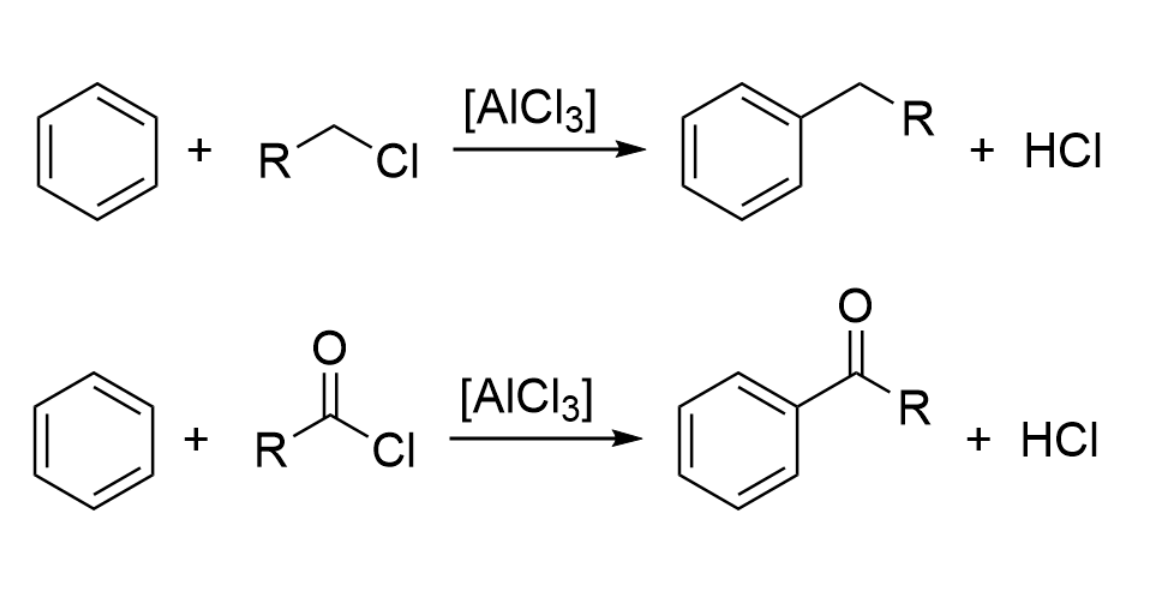
The reactions proceed via an electrophilic aromatic substitution (SEAr), in which AlCl3 first reacts with the respective chloride, forming a carbocation or acylium cation and [AlCl4]–. The cation reacts as an electrophile with the aromatic ring, forming an arenium ion as an intermediate. Deprotonation then gives the desired coupling product, HCl is formed, and AlCl3 is regenerated.
Friedel carried on working on both chemistry and mineralogical research until his death. Charles Friedel is the answer to Guess the Chemist (148).
References
[1] Nekrolog: Charles Friedel,
A. Ladenburg,
Ber. Dtsch. Chem. Ges. 1899, 82, 3721.
[2] Charles Friedel,
M. Bauer,
Centralbl. Mineral. Geol. Paläontol. 1900, 53.
[3] Friedel Memorial Lecture,
J. M. Crafts,
J. Chem. Soc. Trans. 1900, 77, 993–1019.
https://doi.org/10.1039/CT9007700993
[4] Charles Friedel (1832–1899),
A. Willemart,
J. Chem. Educ. 1949, 26, 3.
https://doi.org/10.1021/ed026p3
[5] Sur quelques nouvelles combinations organiques du silicium et sur le poids atomique de cet élement,
C. Friedel, J. M. Crafts,
Compt. Rend. 1863, 56, 590.
[6] Sur une nouvelle méthode générale de synthèse d’hydrocarbures, d’acétones, etc.,
C. Friedel, J. M. Crafts,
Compt. Rend. 1877, 84, 1392–1395.
[7] Sur une nouvelle méthode générale de synthèse d’hydrocarbures, d’acétones, etc. II,
C. Friedel, J. M. Crafts,
Compt. Rend. 1877, 84, 1450–1454.
[8] Earliest History of the Friedel-Crafts Reaction,
A. A. Ashdown,
Ind. Eng. Chem. 1927, 19, 1063–1065.
https://doi.org/10.1021/ie50213a037



