Nitroaromatic compounds are useful synthetic intermediates. They are commonly prepared via direct electrophilic aromatic nitration reactions, but this type of reaction can require harsh conditions and have a lack of selectivity and functional group tolerance. Protocols for regiospecific aromatic nitration reactions under milder conditions are, thus, interesting research targets.
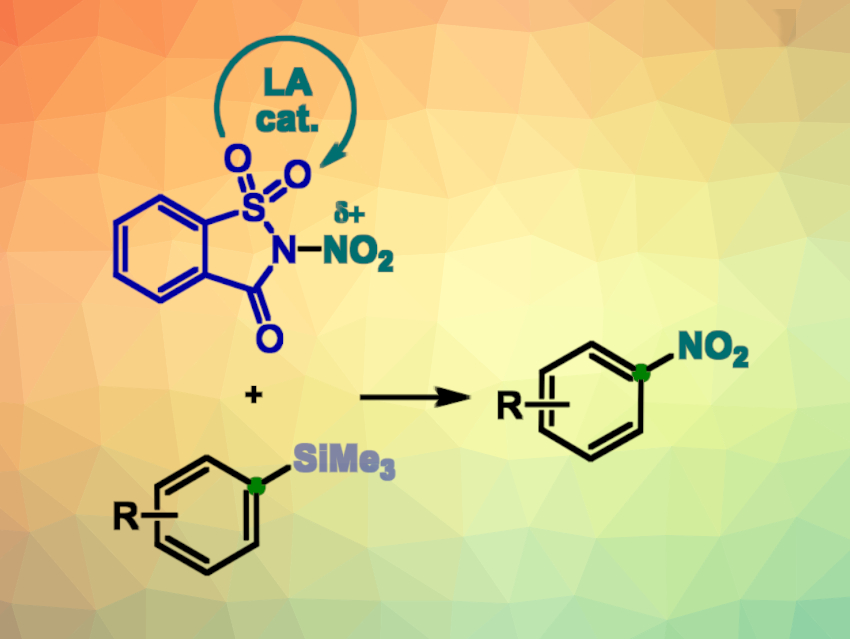
Kai E. O. Ylijoki, Saint Mary’s University, Halifax, Canada, Dmitry Katayev, University of Fribourg, Switzerland, and University of Bern, Switzerland, and colleagues have developed a mild and efficient method for the electrophilic ipso-nitration of organosilanes (pictured). The reaction is enabled by N-nitrosaccharin (pictured top left), an easily accessible, inexpensive, and recyclable reagent that delivers controllable amounts of nitronium species. The team reacted different phenyl trisubstituted silanes with N-nitrosaccharin in the presence of catalytic amounts of magnesium triflate [Mg(OTf)2] in acetonitrile at 85 °C .
The desired nitroaromatic compounds were obtained in yields up to 98 %. The method provides a high level of chemoselectivity and a good functional group tolerance. Various electron-donating and electron-withdrawing aryl substituents at the ortho-, meta-, and para-positions, as well as heteroatomic substrates, were well tolerated. In addition, the reaction conditions complement those of other ipso-substitutions, allowing the modular construction of complex organic molecules.
- Catalytic ipso‐Nitration of Organosilanes Enabled by Electrophilic N‐Nitrosaccharin Reagent,
Ivan Mosiagin, Anthony J. Fernandes, Alena Budinska, Liana Hayriyan, Kai E. O. Ylijoki, Dmitry Katayev,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202310851


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
